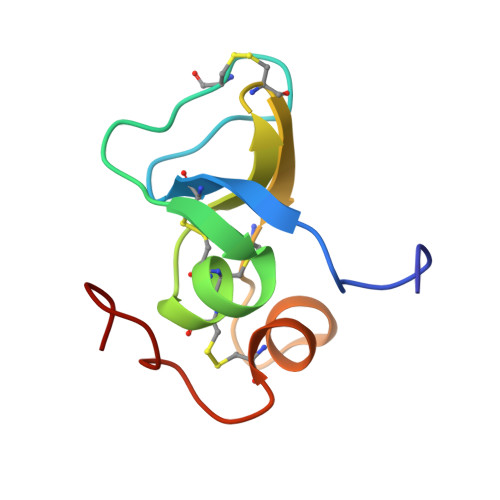
Solution Structure of the Third TB Domain from LTBP1 Provides Insight into Assembly of the Large Latent Complex that Sequesters Latent TGF-beta.
Lack, J., O'leary, J.M., Knott, V., Yuan, X., Rifkin, D.B., Handford, P.A., Downing, A.K.(2003) J Mol Biol 334: 281-291
- PubMed: 14607119
- DOI: https://doi.org/10.1016/j.jmb.2003.09.053
- Primary Citation of Related Structures:
1KSQ - PubMed Abstract:
Almost all TGF-beta is secreted as part of a large latent complex. This complex is formed from three molecules, a latent transforming growth factor-beta binding protein (LTBP), which plays roles in targeting and activation, a latency associated peptide (LAP), which regulates latency, and the TGF-beta cytokine. LAP is the TGF-beta pro-peptide that is cleaved intracellularly prior to secretion, and TGF-beta binds non-covalently to LAP. Formation of the large latent complex is important for the efficient secretion of TGF-beta. Previous studies have revealed that the LTBP-LAP interaction is mediated by intracellular exchange of a single disulphide bond within the third, and only the third, TB domain (TB3) with LAP. We have previously reported the structure of a homologous TB domain from fibrillin-1. However, TB3 contains a two amino acid insertion, not found in fibrillin-1 TB domains, which is not amenable to molecular modelling. In order to clarify the basis of TB domain function, we have determined the solution NMR structure of TB3(LTBP1). Comparison with the fibrillin-1 TB domain reveals that the two-residue insertion is associated with a significant increase in solvent accessibility of one of the disulphide bonds (linking the second and sixth cysteine residues). Site-directed mutagenesis and NMR studies indicate that this is the only disulphide bond that can be removed without perturbing the TB domain fold. Furthermore, a ring of negatively charged residues has been identified that surrounds this disulphide bond. Homology modelling suggests that the surface properties of TB3 domains from different LTBP isoforms correlate with binding activities. This research provides testable hypotheses regarding the molecular basis of complex formation between LTBPs and LAPs.
Organizational Affiliation:
Division of Structural Biology, Department of Biochemistry, University of Oxford, South Parks Road, Oxford OX1 3QU, UK.