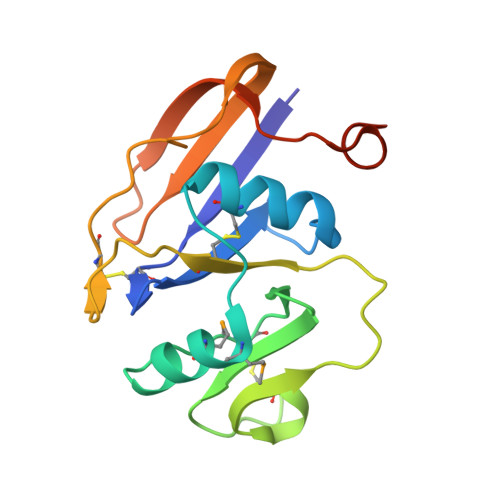
Structure of the Regulatory Hyaluronan-Binding Domain in the Inflammatory Leukocyte Homing Receptor Cd44
Teriete, P., Banerji, S., Noble, M., Blundell, C., Wright, A., Pickford, A., Lowe, E., Mahoney, D., Tammi, M., Kahmann, J., Campbell, I., Day, A., Jackson, D.(2004) Mol Cell 13: 483
- PubMed: 14992719
- DOI: https://doi.org/10.1016/s1097-2765(04)00080-2
- Primary Citation of Related Structures:
1POZ, 1UUH - PubMed Abstract:
Adhesive interactions involving CD44, the cell surface receptor for hyaluronan, underlie fundamental processes such as inflammatory leukocyte homing and tumor metastasis. Regulation of such events is critical and appears to be effected by changes in CD44 N-glycosylation that switch the receptor "on" or "off" under appropriate circumstances. How altered glycosylation influences binding of hyaluronan to the lectin-like Link module in CD44 is unclear, although evidence suggests additional flanking sequences peculiar to CD44 may be involved. Here we show using X-ray crystallography and NMR spectroscopy that these sequences form a lobular extension to the Link module, creating an enlarged HA binding domain and a formerly unidentified protein fold. Moreover, the disposition of key N-glycosylation sites reveals how specific sugar chains could alter both the affinity and avidity of CD44 HA binding. Our results provide the necessary structural framework for understanding the diverse functions of CD44 and developing novel therapeutic strategies.
Organizational Affiliation:
Medical Research Council Human Immunology Unit, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, Headington, Oxford OX3 9DS, United Kingdom.