Solution Structure of Human Proguanylin: The Role of a Hormone Prosequence
Lauber, T., Neudecker, P., Roesch, P., Marx, U.C.(2003) J Biol Chem 278: 24118
- PubMed: 12707255
- DOI: https://doi.org/10.1074/jbc.M300370200
- Primary Citation of Related Structures:
1O8R - PubMed Abstract:
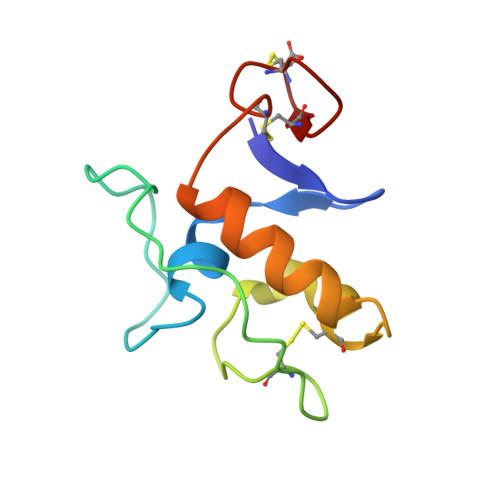
The endogenous ligand of guanylyl cyclase C, guanylin, is produced as the 94-amino-acid prohormone proguanylin, with the hormone guanylin located at the COOH terminus of the prohormone. The solution structure of proguanylin adopts a new protein fold and consists of a three-helix bundle, a small three-stranded beta-sheet of two NH2-terminal strands and one COOH-terminal strand, and an unstructured linker region. The sequence corresponding to guanylin is fixed in its bioactive topology and is involved in interactions with the NH2-terminal beta-hairpin: the hormone region (residues 80-94) partly wraps around the first 4 NH2-terminal residues that thereby shield parts of the hormone surface. These interactions provide an explanation for the negligible bioactivity of the prohormone as well as the important role of the NH2-terminal residues in the disulfide-coupled folding of proguanylin. Since the ligand binding region of guanylyl cyclase C is predicted to be located around an exposed beta-strand, the intramolecular interactions observed between guanylin and its prosequence may be comparable with the guanylin/receptor interaction.
Organizational Affiliation:
Lehrstuhl für Biopolymere, Universität Bayreuth, Universitätstrasse 30, 95447 Bayreuth, Germany.