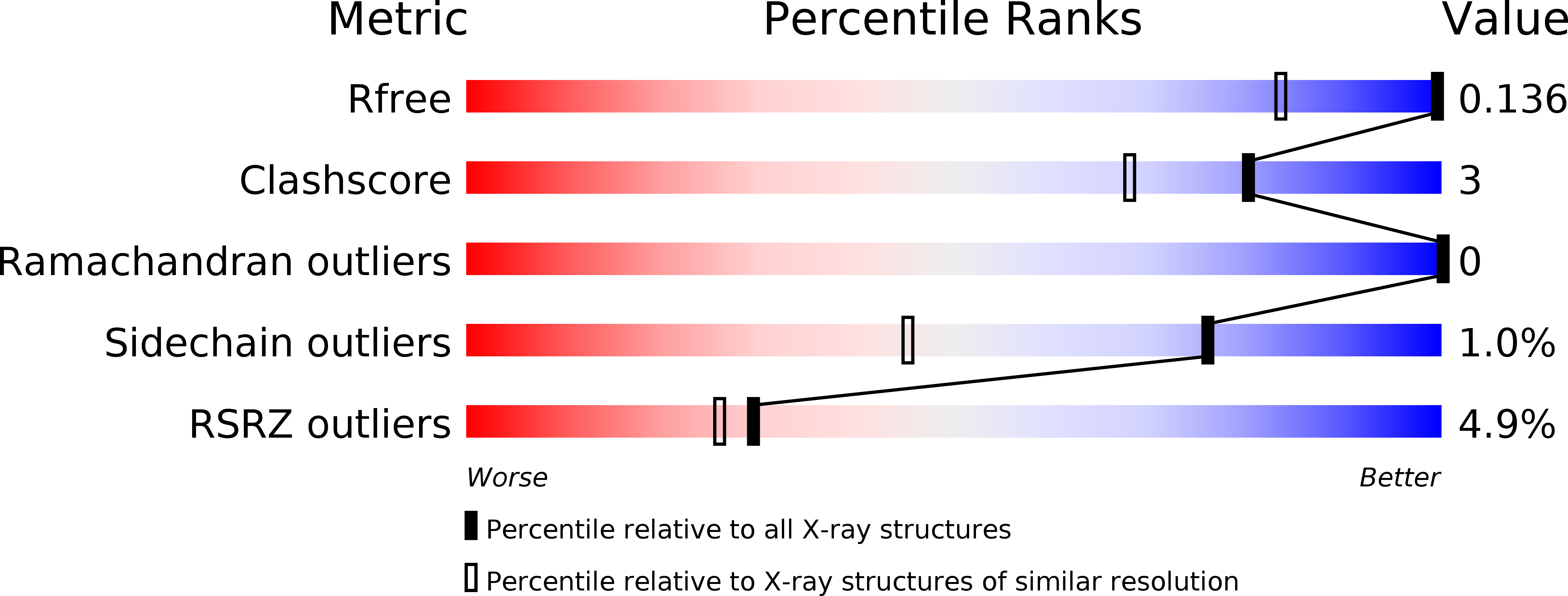
Diacritic Binding of an Indenoindole Inhibitor by CK2 alpha Paralogs Explored by a Reliable Path to Atomic Resolution CK2 alpha ' Structures.
Lindenblatt, D., Nickelsen, A., Applegate, V.M., Hochscherf, J., Witulski, B., Bouaziz, Z., Marminon, C., Bretner, M., Le Borgne, M., Jose, J., Niefind, K.(2019) ACS Omega 4: 5471-5478
- PubMed: 31559376
- DOI: https://doi.org/10.1021/acsomega.8b03415
- Primary Citation of Related Structures:
6HBN, 6HMB, 6HMC, 6HMD, 6HME, 6HMQ - PubMed Abstract:
CK2α and CK2α' are the two isoforms of the catalytic subunit of human protein kinase CK2, an important target for cancer therapy. They have similar, albeit not identical functional and structural properties, and were occasionally reported to be inhibited with distinct efficacies by certain ATP-competitive ligands. Here, we present THN27, an indeno[1,2- b ]indole derivative, as a further inhibitor with basal isoform selectivity. The selectivity disappears when measured using CK2α/CK2α' complexes with CK2β, the regulatory CK2 subunit. Co-crystal structures of THN27 with CK2α and CK2α' reveal that subtle differences in the conformational variability of the interdomain hinge region are correlated with the observed effect. In the case of CK2α', a crystallographically problematic protein so far, this comparative structural analysis required the development of an experimental strategy that finally enables atomic resolution structure determinations with ab initio phasing of potentially any ATP-competitive CK2 inhibitor and possibly many non-ATP-competitive ligands as well bound to CK2α'.
Organizational Affiliation:
Department für Chemie, Institut für Biochemie, Universität zu Köln, Zülpicher Straße 47, D-50674 Köln, Germany.