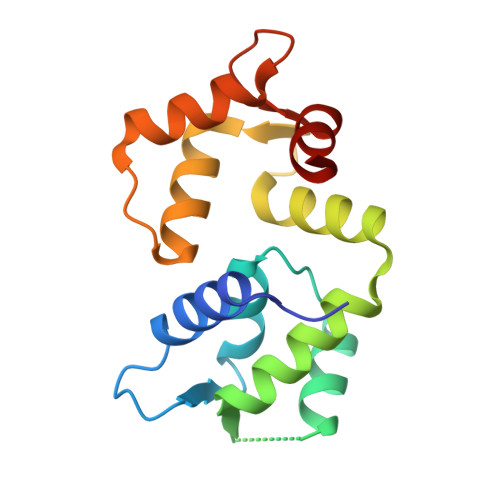
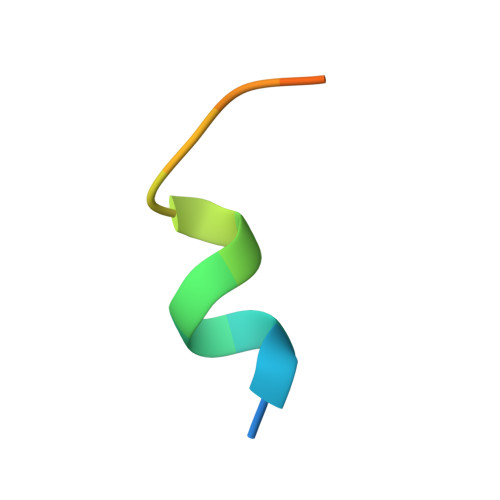
Molecular basis of AKAP79 regulation by calmodulin.
Patel, N., Stengel, F., Aebersold, R., Gold, M.G.(2017) Nat Commun 8: 1681-1681
- PubMed: 29162807
- DOI: https://doi.org/10.1038/s41467-017-01715-w
- Primary Citation of Related Structures:
5NIN - PubMed Abstract:
AKAP79/150 is essential for coordinating second messenger-responsive enzymes in processes including synaptic long-term depression. Ca 2+ directly regulates AKAP79 through its effector calmodulin (CaM), but the molecular basis of this regulation was previously unknown. Here, we report that CaM recognizes a '1-4-7-8' pattern of hydrophobic amino acids starting at Trp79 in AKAP79. Cross-linking coupled to mass spectrometry assisted mapping of the interaction site. Removal of the CaM-binding sequence in AKAP79 prevents formation of a Ca 2+ -sensitive interface between AKAP79 and calcineurin, and increases resting cellular PKA phosphorylation. We determined a crystal structure of CaM bound to a peptide encompassing its binding site in AKAP79. CaM adopts a highly compact conformation in which its open Ca 2+ -activated C-lobe and closed N-lobe cooperate to recognize a mixed α/3 10 helix in AKAP79. The structure guided a bioinformatic screen to identify potential sites in other proteins that may employ similar motifs for interaction with CaM.
Organizational Affiliation:
Department of Neuroscience, Physiology & Pharmacology, University College London, Gower Street, London, WC1E 6BT, UK.