Crystal structure of s-adenosylhomocysteine nucleosidase from streptococcus pyogenes in complex with 5-methylthiotubericidin;
Ponniah, K., Norris, G.E., Anderson, B.F., Brown, R.L., Tyler, P.C., Evans, G.B., Frohlich, R.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| MTA/SAH nucleosidase | 236 | Streptococcus pyogenes ATCC 10782 | Mutation(s): 0 Gene Names: HMPREF0841_1463, mtn N, mtnN EC: 3.2.2.16 (PDB Primary Data), 3.2.2.9 (PDB Primary Data) | 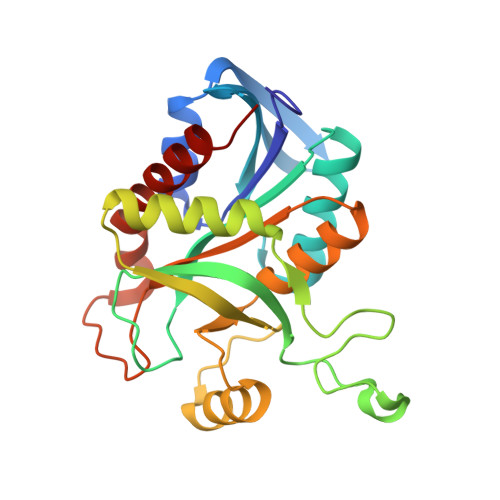 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MTA Query on MTA | C [auth B] | 5'-DEOXY-5'-METHYLTHIOADENOSINE C11 H15 N5 O3 S WUUGFSXJNOTRMR-IOSLPCCCSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 49.735 | α = 90 |
| b = 72.345 | β = 90 |
| c = 116.246 | γ = 90 |
| Software Name | Purpose |
|---|---|
| CrystalClear | data collection |
| PHASER | phasing |
| REFMAC | refinement |
| MOSFLM | data reduction |
| SCALA | data scaling |