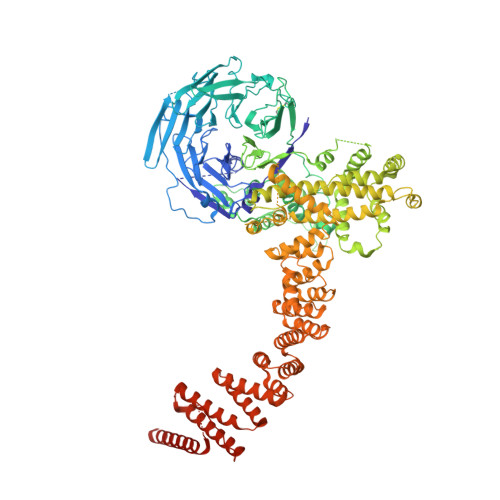
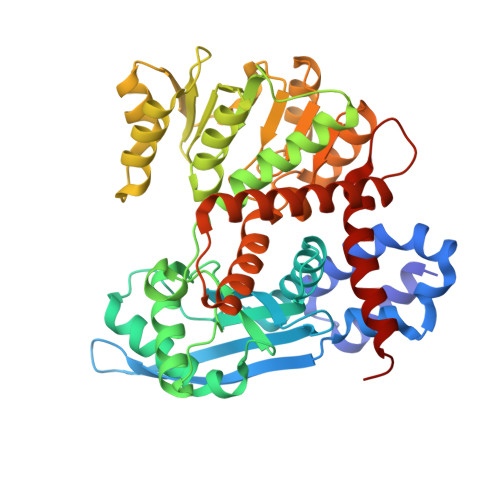
Molecular basis for Nup37 and ELY5/ELYS recruitment to the nuclear pore complex.
Bilokapic, S., Schwartz, T.U.(2012) Proc Natl Acad Sci U S A 109: 15241-15246
- PubMed: 22955883
- DOI: https://doi.org/10.1073/pnas.1205151109
- Primary Citation of Related Structures:
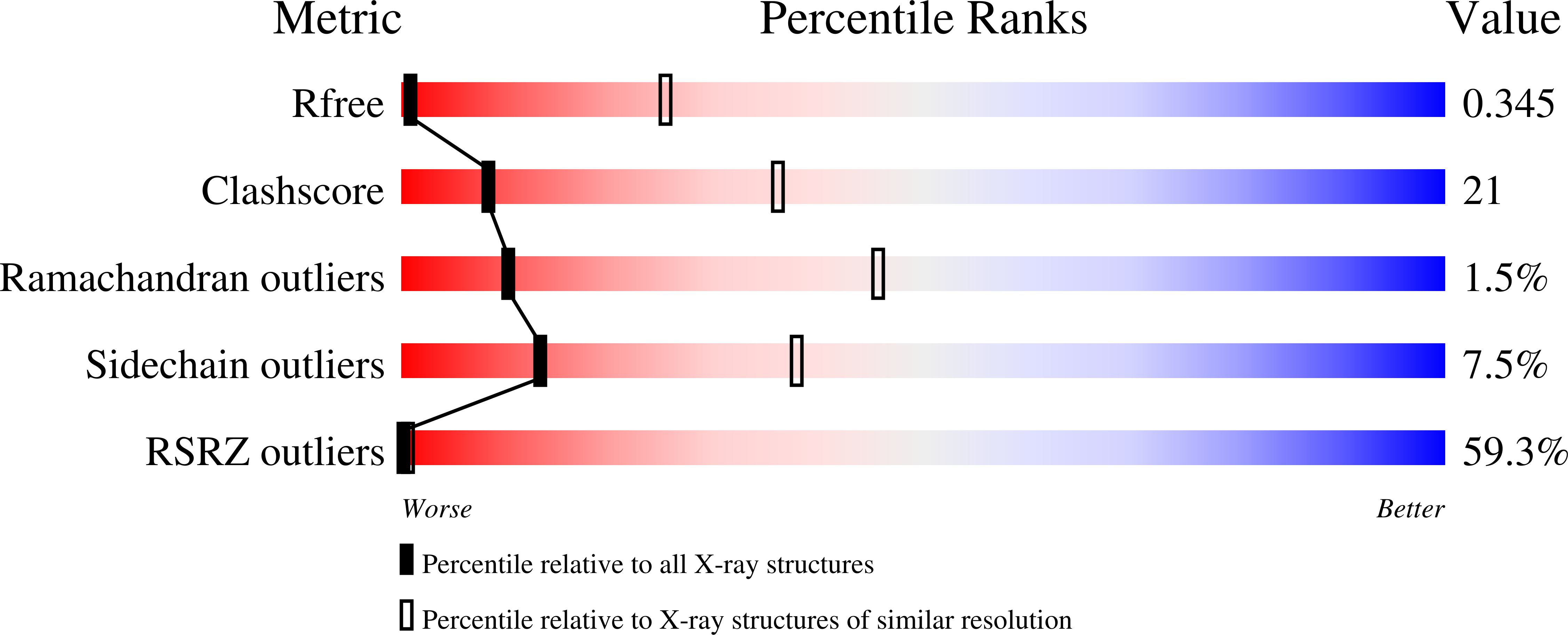
4FCC, 4FHL, 4FHM, 4FHN - PubMed Abstract:
Nucleocytoplasmic transport is mediated by nuclear pore complexes (NPCs), enormous assemblies composed of multiple copies of ~30 different proteins called nucleoporins. To unravel the basic scaffold underlying the NPC, we have characterized the species-specific scaffold nucleoporin Nup37 and ELY5/ELYS. Both proteins integrate directly via Nup120/160 into the universally conserved heptameric Y-complex, the critical unit for the assembly and functionality of the NPC. We present the crystal structure of Schizosaccharomyces pombe Nup37 in complex with Nup120, a 174-kDa subassembly that forms one of the two short arms of the Y-complex. Nup37 binds near the bend of the L-shaped Nup120 protein, potentially stabilizing the relative orientation of its two domains. By means of reconstitution assays, we pinpoint residues crucial for this interaction. In vivo and in vitro results show that ELY5 binds near an interface of the Nup120-Nup37 complex. Complementary biochemical and cell biological data refine and consolidate the interactions of Nup120 within the current Y-model. Finally, we propose an orientation of the Y-complex relative to the pore membrane, consistent with the lattice model.
Organizational Affiliation:
Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.