Dioxygen activation for the self-degradation of heme: reaction mechanism and regulation of heme oxygenase.
Matsui, T., Iwasaki, M., Sugiyama, R., Unno, M., Ikeda-Saito, M.(2010) Inorg Chem 49: 3602-3609
- PubMed: 20380462
- DOI: https://doi.org/10.1021/ic901869t
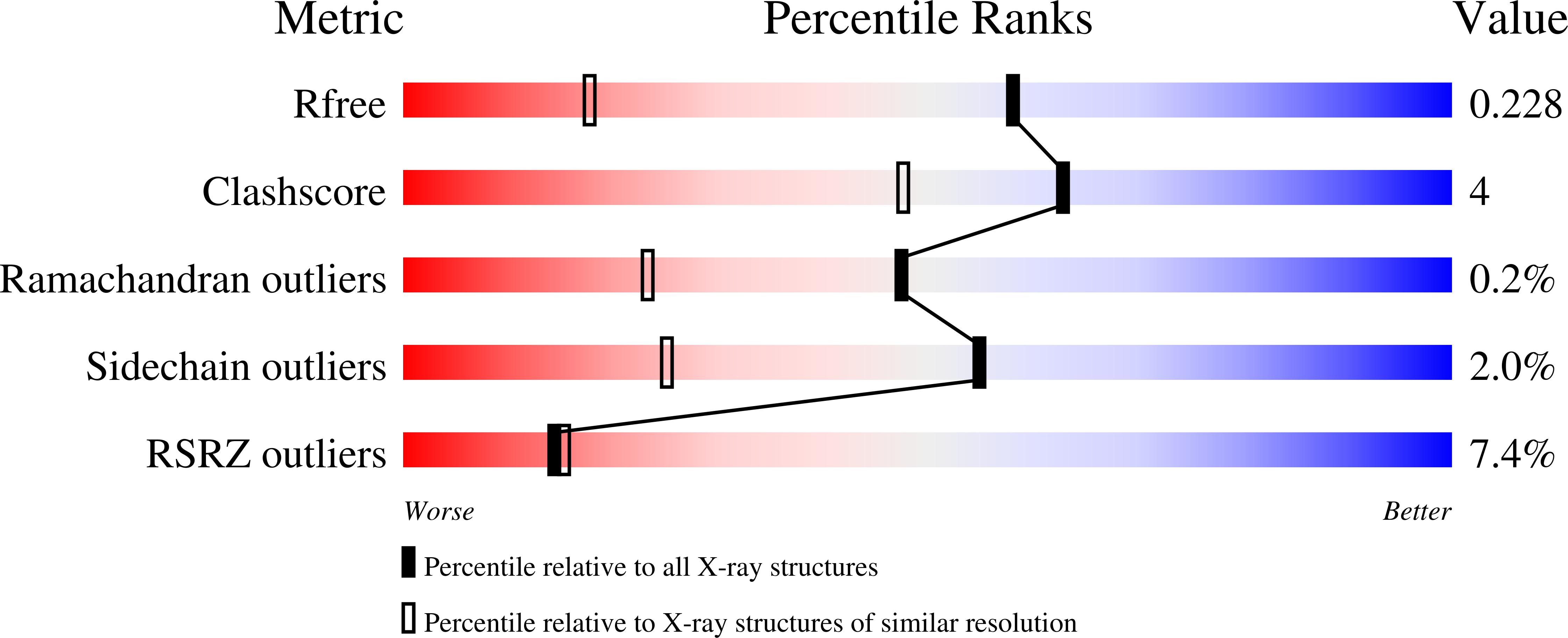
- Primary Citation of Related Structures:
3I8R, 3I9T, 3I9U - PubMed Abstract:
Heme oxygenase (HO) catalyzes the regiospecific conversion of heme to biliverdin, CO, and free iron through three successive oxygenation reactions. HO catalysis is unique in that all three O(2) activations are performed by the substrate itself. This Forum Article overviews our current understanding on the structural and biochemical properties of HO catalysis, especially its first and third oxygenation steps. The HO first step, regiospecific hydroxylation of the porphyrin alpha-meso-carbon atom, is of particular interest because of its sharp contrast to O(2) activation by cytochrome P450. HO was proposed to utilize the FeOOH species but not conventional ferryl hemes as a reactive intermediate for self-hydroxylation. We have succeeded in preparing and characterizing the FeOOH species of HO at low temperature, and our analyses of its reaction, together with mutational and crystallographic studies, reveal that protonation of FeOOH by a distal water molecule is critical in promoting the unique self-hydroxylation. The second oxygenation is a rapid, spontaneous autooxidation of the reactive alpha-meso-hydroxyheme in which the HO enzyme does not play a critical role. Further O(2) activation by verdoheme cleaves its porphyrin macrocycle to form biliverdin and free ferrous iron. This third step has been considered to be a major rate-determining step of HO catalysis to regulate the enzyme activity. Our reaction analysis strongly supports the FeOOH verdoheme as the key intermediate of the ring-opening reaction. This mechanism is very similar to that of the first meso-hydroxylation, and the distal water is suggested to enhance the third step as expected from the similarity. The HO mechanistic studies highlight the catalytic importance of the distal hydrogen-bonding network, and this manuscript also involves our attempts to develop HO inhibitors targeting the unique distal structure.
Organizational Affiliation:
Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, Katahira, Aoba, Sendai 980-8577, Japan. matsui@tagen.tohoku.ac.jp