Structure of human monocyte chemoattractant protein 4 (MCP-4/CCL13).
Barinka, C., Prahl, A., Lubkowski, J.(2008) Acta Crystallogr D Biol Crystallogr 64: 273-278
- PubMed: 18323622
- DOI: https://doi.org/10.1107/S0907444907066164
- Primary Citation of Related Structures:
2RA4 - PubMed Abstract:
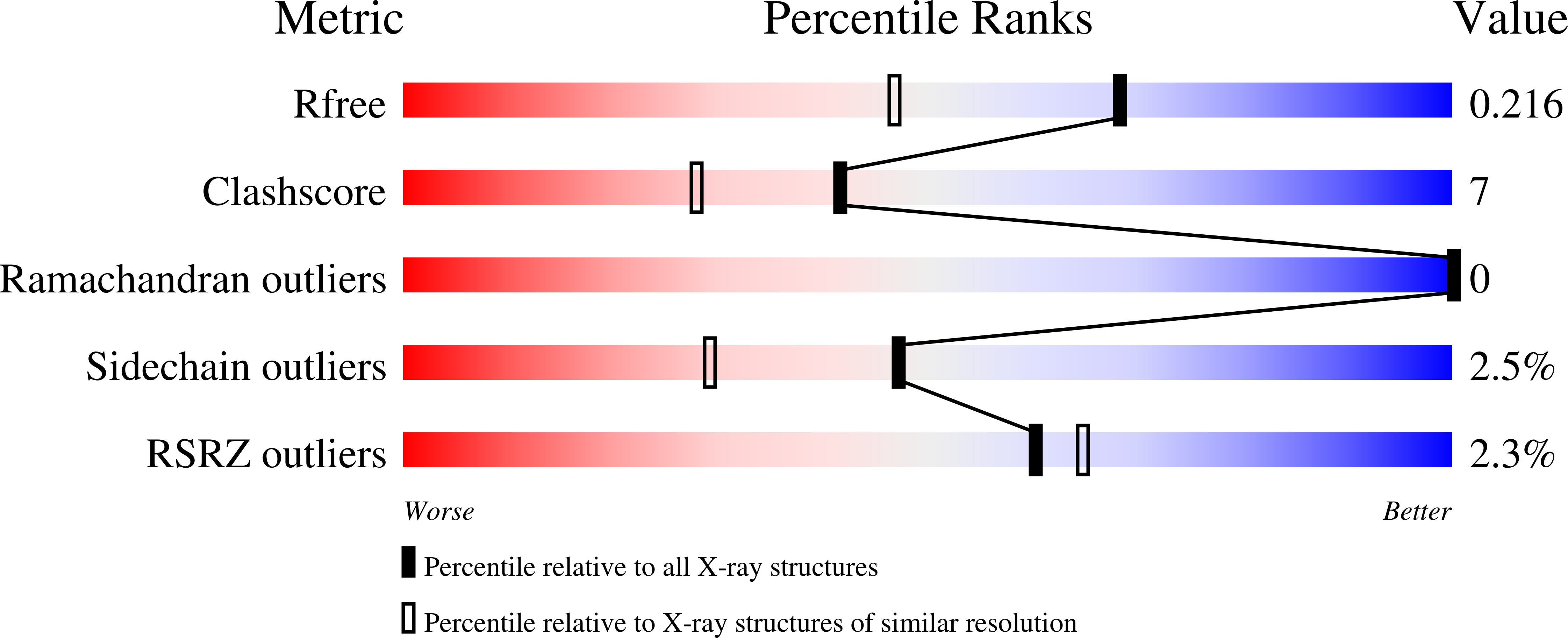
Monocyte chemoattractant proteins (MCPs) belong to the CC chemokine family and are involved in many (patho)physiological processes characterized by mononuclear cell infiltration, including tissue remodeling, atherosclerosis and cancer metastasis. Here, the crystal structure of human monocyte chemoattractant protein 4 (MCP-4) refined at 1.70 A resolution is reported with crystallographic values R = 0.180 and R free = 0.212. The overall MCP-4 fold reveals the typical tertiary features of the CC chemokine family. A central three-stranded antiparallel beta-sheet is C-terminally flanked by an overlaying alpha-helix, while the N-terminal part of the molecule forms an extended loop that is anchored to the rest of the molecule via two disulfide bridges, Cys11-Cys35 and Cys12-Cys51. The crystal packing suggests the existence of MCP-4 dimers with a dimerization interface similar to those previously reported for the X-ray structures of MCP-1 and MCP-2.
Organizational Affiliation:
National Cancer Institute at Frederick, Center for Cancer Research, Frederick, MD 21702, USA.