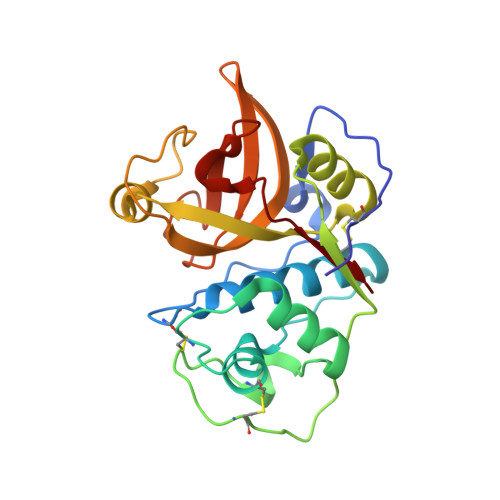
Three-dimensional structure and IgE-binding properties of mature fully active Der p 1, a clinically relevant major allergen
de Halleux, S., Stura, E., VanderElst, L., Carlier, V., Jacquemin, M., Saint-Remy, J.-M.(2006) J Allergy Clin Immunol 117: 571-576
- PubMed: 16522455
- DOI: https://doi.org/10.1016/j.jaci.2005.11.032
- Primary Citation of Related Structures:
2AS8 - PubMed Abstract:
Der p 1 is a 25-kd allergen with cysteine protease activity. Sensitization to Der p 1 affects a large proportion of individuals with allergy, resulting in rhinitis, asthma, and/or atopic dermatitis.
Organizational Affiliation:
Center for Molecular and Vascular Biology, University of Leuven, Belgium.