NMR Solution Structure of Ole e 6, a Major Allergen from Olive Tree Pollen.
Trevino, M.A., Garcia-Mayoral, M.F., Barral, P., Villalba, M., Santoro, J., Rico, M., Rodriguez, R., Bruix, M.(2004) J Biol Chem 279: 39035-39041
- PubMed: 15247256
- DOI: https://doi.org/10.1074/jbc.M406045200
- Primary Citation of Related Structures:
1SS3 - PubMed Abstract:
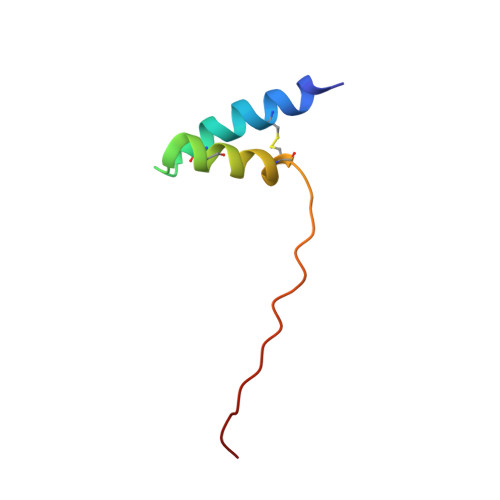
Ole e 6 is a pollen protein from the olive tree (Olea europaea) that exhibits allergenic activity with a high prevalence among olive-allergic individuals. The three-dimensional structure of Ole e 6 has been determined in solution by NMR methods. This is the first experimentally determined structure of an olive tree pollen allergen. The structure of this 50-residue protein is based on 486 upper limit distance constraints derived from nuclear Overhauser effects and 24 torsion angle restraints. The global fold of Ole e 6 consists of two nearly antiparallel alpha-helices, spanning residues 3-19 and 23-33, that are connected by a short loop and followed by a long, unstructured C-terminal tail. Viewed edge-on, the structured N terminus has a dumbbell-like shape with the two helices on the outside and with the hydrophobic core, mainly composed of 3 aromatic and 6 cysteine residues, on the inside. All the aromatic rings lie on top of and pack against the three disulfide bonds. The lack of thermal unfolding, even at 85 degrees C, indicates a high conformational stability. Based on the analysis of the molecular surface, we propose five plausible epitopes for IgE recognition. The results presented here provide the structural foundation for future experiments to verify the antigenicity of the proposed epitopes, as well as to design novel hypoallergenic forms of the protein suitable for diagnosis and treatment of type-I allergies. In addition, three-dimensional structure features of Ole e 6 are discussed to provide a basis for future functional studies.
Organizational Affiliation:
Departamento de Espectroscopía y Estructura Molecular, Instituto de Química Física "Rocasolano," Consejo Superior de Investigaciones Científicas, Serrano 119, 28006 Madrid, Spain.