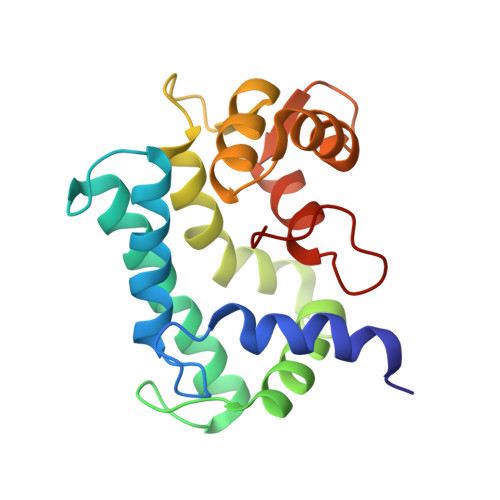
NMR solution structure of calerythrin, an EF-hand calcium-binding protein from Saccharopolyspora erythraea
Tossavainen, H., Permi, P., Annila, A., Kilpelainen, I., Drakenberg, T.(2003) Eur J Biochem 270: 2505-2512
- PubMed: 12755706
- DOI: https://doi.org/10.1046/j.1432-1033.2003.03623.x
- Primary Citation of Related Structures:
1NYA - PubMed Abstract:
The structure of calerythrin, a prokaryotic 20 kDa calcium-binding protein has been determined by solution NMR spectroscopy. Distance, dihedral angle, J coupling, secondary chemical shift, residual dipolar coupling and radius of gyration restraints reveal four EF-hand motifs arranged in a compact globular structure. A tight turn in the middle of the amino acid sequence brings the two halves, each comprising a pair of EF-hands, close together. The structural similarity between calerythrin and the eukaryotic sarcoplasmic calcium-binding proteins is notable.
Organizational Affiliation:
NMR laboratory, Structural Biology and Biophysics, Institute of Biotechnology, University of Helsinki, Finland.