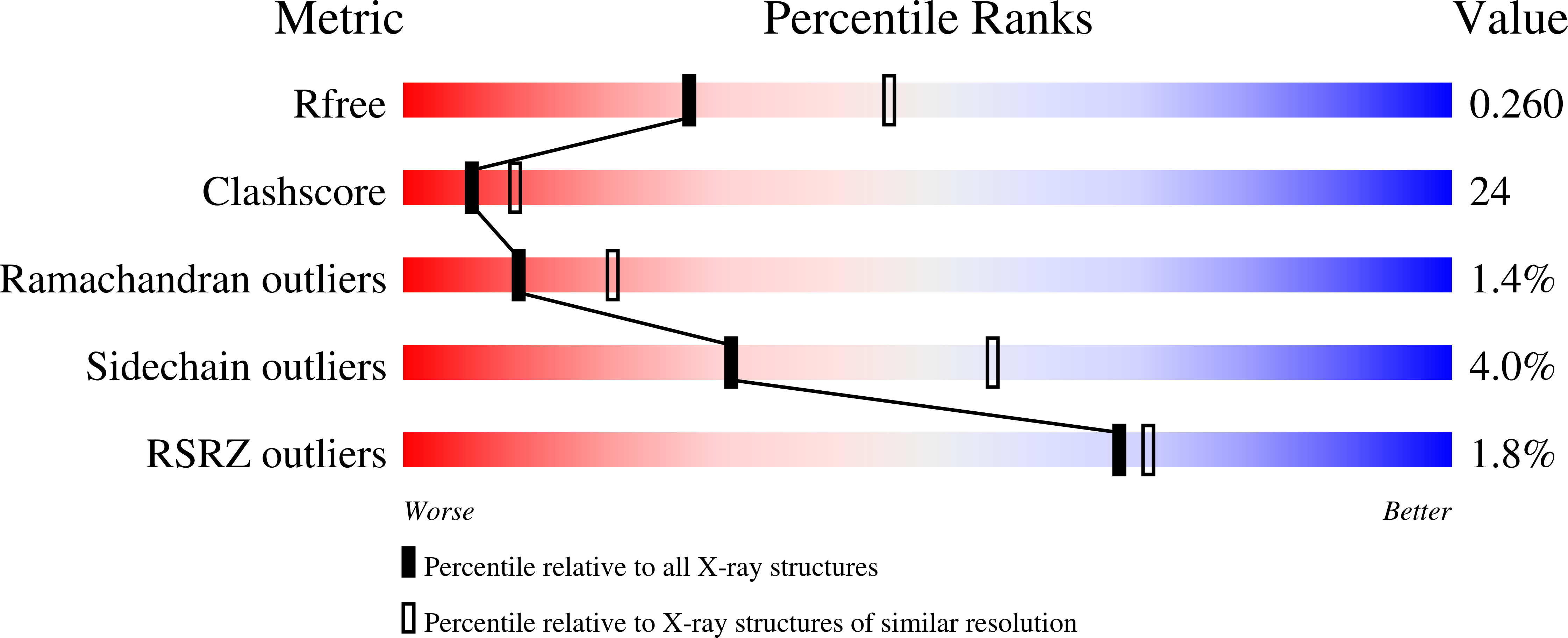
Structure of Avian AICAR Transformylase with a Multisubstrate Adduct Inhibitor beta-DADF Identifies the Folate Binding Site.
Wolan, D.W., Greasley, S.E., Wall, M.J., Benkovic, S.J., Wilson, I.A.(2003) Biochemistry 42: 10904-10914
- PubMed: 12974624
- DOI: https://doi.org/10.1021/bi030106h
- Primary Citation of Related Structures:
1OZ0 - PubMed Abstract:
The penultimate catalytic step of the purine de novo synthesis pathway is the conversion of aminoimidazole-4-carboxamide ribonucleotide (AICAR) to 5-formyl-AICAR that requires the cofactor N(10)-formyl-tetrahydrofolate as the formyl donor. This reaction is catalyzed by the AICAR transformylase domain of the bifunctional enzyme AICAR transformylase/inosine monophosphate cyclohydrolase (ATIC). Identification of the location of the AICAR transformylase active site was previously elucidated from the crystal structure of the avian ATIC with bound substrate AICAR; however, due to the absence of any bound folate, the folate binding region of the active site could not be identified. Here, we have determined the homodimeric crystal structure of avian ATIC in complex with the ATIC-specific multisubstrate adduct inhibitor beta-DADF to 2.5 A resolution. Beta-DADF encompasses both the AICAR and folate moieties into a single covalently linked entity, thereby allowing for the characterization of the folate binding pocket of the AICAR transformylase active site. Beta-DADF is intimately bound at the dimer interface of the transformylase domains with the majority of AICAR moiety interactions occurring within one subunit, whereas the primary interactions to the folate occur with the opposing subunit. The crystal structure suggests that a buried Lys(267) is transiently protonated during formyl transfer allowing for the stabilization of the oxyanion transition state and subsequent protonation of N10 on the tetrahydrofolate leaving group. Furthermore, the beta-DADF-bound structure provides a more optimal three-dimensional scaffold to improve the design of specific antineoplastic agents.
Organizational Affiliation:
The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA.