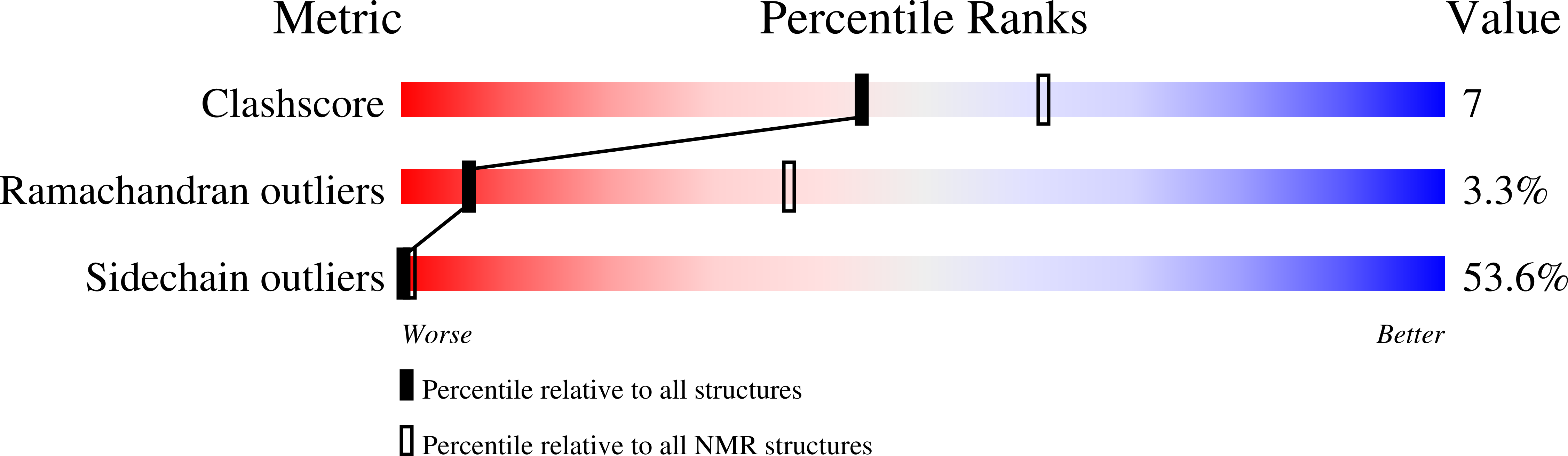NMR identification and characterization of the flexible regions in the 160 kDa molten globule-like aggregate of barstar at low pH.
Juneja, J., Bhavesh, N.S., Udgaonkar, J.B., Hosur, R.V.(2002) Biochemistry 41: 9885-9899
- PubMed: 12146954
- DOI: https://doi.org/10.1021/bi026034w
- Primary Citation of Related Structures:
1L1K - PubMed Abstract:
Barstar is known to form a molten globule-like A form below pH 4. This form exists as a soluble aggregate of 16 monomeric subunits, and appears to remain homogeneous in solution for at least two weeks. Here, structural characterization by NMR of the flexible regions in the A form of barstar has been carried out at pH 2.7 and 25 degrees C. Significantly, the A form appears to be a symmetrical aggregate. Using the recently described fast assignment strategy from HNN and HN(C)N spectra, along with the standard triple resonance and three-dimensional NMR experiments, the flexible segment of the aggregate has been identified to belong largely to the N-terminal end of the polypeptide chain; sequential connectivities were obtained for the first 20 residues (except two) from these experiments. This segment is free in each of the monomeric subunits, and does not form a part of the aggregated core of the A form. The secondary chemical shifts of these residues suggest propensity toward an extended structure. Their (3)J(HN,H)(alpha) coupling constants have values corresponding to those in a random coil structure. However, a few medium-range NOEs, some of them involving side chain atoms, are observed between some residues in this segment. The lowered temperature coefficients of the H(N) chemical shifts compared to random coil values indicate possibilities of some hydrogen bonding in this region. Analysis of the (15)N relaxation parameters and reduced spectral density functions, in particular the negative values of heteronuclear NOEs, indicates large-amplitude high-frequency motions in the N-terminal segments; the first three residues show more negative NOEs than the others. The (15)N transverse relaxation rates and the J(0) spectral density values for residues Ser12 and Ser69 are significantly larger than for the rest, indicating some microsecond to millisecond time scale conformational exchange contributions to the relaxation of these residues. Taken all together, the data suggest that the A form of barstar is an aggregate with a rigid core, but with the N-terminal 20 residues of each of the monomeric subunits, in a highly dynamic random coil conformation which shows transient local ordering of structure. The N-terminal segment, anchored to the aggregated core, exhibits free-flight motion.
Organizational Affiliation:
National Centre for Biological Sciences, Tata Institute of Fundamental Research, GKVK Campus, Bangalore 560 065, India.