NMR Spectroscopy Reveals Common Structural Features of the Birch Pollen Allergen Bet v 1 and the cherry allergen Pru a 1
Schweimer, K., Sticht, H., Boehm, M., Roesch, P.(1999) Appl Magn Reson 17: 449-464
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(1999) Appl Magn Reson 17: 449-464
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| PROTEIN (MAJOR POLLEN ALLERGEN BET V 1-A) | 159 | Betula pendula | Mutation(s): 1 | 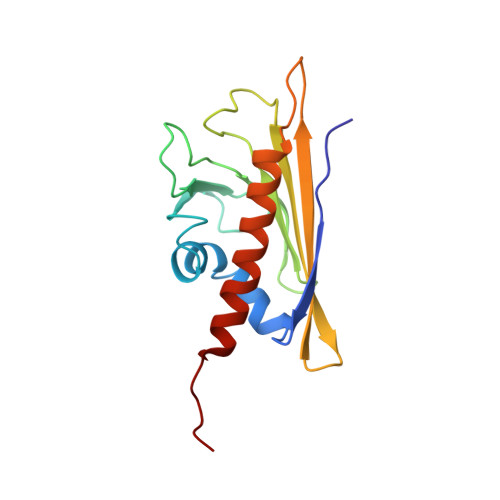 | |
UniProt | |||||
Find proteins for P15494 (Betula pendula) Explore P15494 Go to UniProtKB: P15494 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P15494 | ||||
Sequence AnnotationsExpand | |||||
| |||||