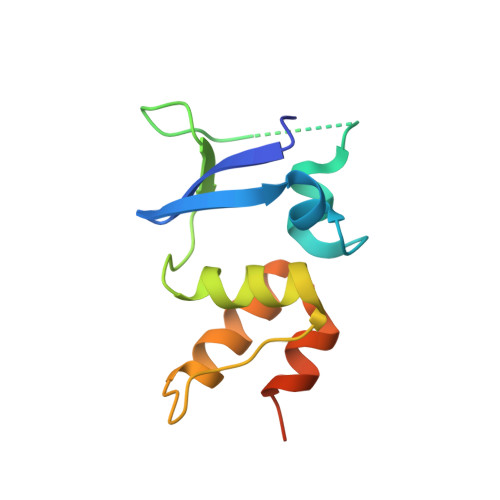
Structural basis for auxiliary subunit KCTD16 regulation of the GABABreceptor.
Zuo, H., Glaaser, I., Zhao, Y., Kurinov, I., Mosyak, L., Wang, H., Liu, J., Park, J., Frangaj, A., Sturchler, E., Zhou, M., McDonald, P., Geng, Y., Slesinger, P.A., Fan, Q.R.(2019) Proc Natl Acad Sci U S A 116: 8370-8379
- PubMed: 30971491
- DOI: https://doi.org/10.1073/pnas.1903024116
- Primary Citation of Related Structures:
6OCP, 6OCR, 6OCT - PubMed Abstract:
Metabotropic GABA B receptors mediate a significant fraction of inhibitory neurotransmission in the brain. Native GABA B receptor complexes contain the principal subunits GABA B1 and GABA B2 , which form an obligate heterodimer, and auxiliary subunits, known as potassium channel tetramerization domain-containing proteins (KCTDs). KCTDs interact with GABA B receptors and modify the kinetics of GABA B receptor signaling. Little is known about the molecular mechanism governing the direct association and functional coupling of GABA B receptors with these auxiliary proteins. Here, we describe the high-resolution structure of the KCTD16 oligomerization domain in complex with part of the GABA B2 receptor. A single GABA B2 C-terminal peptide is bound to the interior of an open pentamer formed by the oligomerization domain of five KCTD16 subunits. Mutation of specific amino acids identified in the structure of the GABA B2 -KCTD16 interface disrupted both the biochemical association and functional modulation of GABA B receptors and G protein-activated inwardly rectifying K + channel (GIRK) channels. These interfacial residues are conserved among KCTDs, suggesting a common mode of KCTD interaction with GABA B receptors. Defining the binding interface of GABA B receptor and KCTD reveals a potential regulatory site for modulating GABA B -receptor function in the brain.
Organizational Affiliation:
Department of Pharmacology, Columbia University, New York, NY 10032.