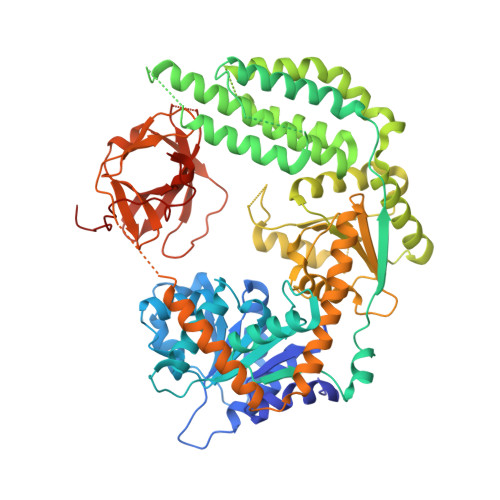
Structural basis of RNA recognition and activation by innate immune receptor RIG-I.
Jiang, F., Ramanathan, A., Miller, M.T., Tang, G.Q., Gale, M., Patel, S.S., Marcotrigiano, J.(2011) Nature 479: 423-427
- PubMed: 21947008
- DOI: https://doi.org/10.1038/nature10537
- Primary Citation of Related Structures:
5E3H - PubMed Abstract:
Retinoic-acid-inducible gene-I (RIG-I; also known as DDX58) is a cytoplasmic pathogen recognition receptor that recognizes pathogen-associated molecular pattern (PAMP) motifs to differentiate between viral and cellular RNAs. RIG-I is activated by blunt-ended double-stranded (ds)RNA with or without a 5'-triphosphate (ppp), by single-stranded RNA marked by a 5'-ppp and by polyuridine sequences. Upon binding to such PAMP motifs, RIG-I initiates a signalling cascade that induces innate immune defences and inflammatory cytokines to establish an antiviral state. The RIG-I pathway is highly regulated and aberrant signalling leads to apoptosis, altered cell differentiation, inflammation, autoimmune diseases and cancer. The helicase and repressor domains (RD) of RIG-I recognize dsRNA and 5'-ppp RNA to activate the two amino-terminal caspase recruitment domains (CARDs) for signalling. Here, to understand the synergy between the helicase and the RD for RNA binding, and the contribution of ATP hydrolysis to RIG-I activation, we determined the structure of human RIG-I helicase-RD in complex with dsRNA and an ATP analogue. The helicase-RD organizes into a ring around dsRNA, capping one end, while contacting both strands using previously uncharacterized motifs to recognize dsRNA. Small-angle X-ray scattering, limited proteolysis and differential scanning fluorimetry indicate that RIG-I is in an extended and flexible conformation that compacts upon binding RNA. These results provide a detailed view of the role of helicase in dsRNA recognition, the synergy between the RD and the helicase for RNA binding and the organization of full-length RIG-I bound to dsRNA, and provide evidence of a conformational change upon RNA binding. The RIG-I helicase-RD structure is consistent with dsRNA translocation without unwinding and cooperative binding to RNA. The structure yields unprecedented insight into innate immunity and has a broader impact on other areas of biology, including RNA interference and DNA repair, which utilize homologous helicase domains within DICER and FANCM.
Organizational Affiliation:
Center for Advanced Biotechnology and Medicine, Department of Chemistry and Chemical Biology, Rutgers University, 679 Hoes Lane West, Piscataway, New Jersey 08854, USA.