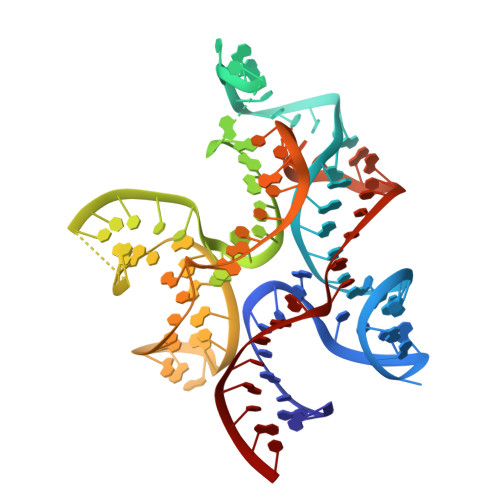
Crystal structure of a c-di-AMP riboswitch reveals an internally pseudo-dimeric RNA.
Jones, C.P., Ferre-D'Amare, A.R.(2014) EMBO J 33: 2692-2703
- PubMed: 25271255
- DOI: https://doi.org/10.15252/embj.201489209
- Primary Citation of Related Structures:
4W90, 4W92 - PubMed Abstract:
Cyclic diadenosine monophosphate (c-di-AMP) is a second messenger that is essential for growth and homeostasis in bacteria. A recently discovered c-di-AMP-responsive riboswitch controls the expression of genes in a variety of bacteria, including important pathogens. To elucidate the molecular basis for specific binding of c-di-AMP by a gene-regulatory mRNA domain, we have determined the co-crystal structure of this riboswitch. Unexpectedly, the structure reveals an internally pseudo-symmetric RNA in which two similar three-helix-junction elements associate head-to-tail, creating a trough that cradles two c-di-AMP molecules making quasi-equivalent contacts with the riboswitch. The riboswitch selectively binds c-di-AMP and discriminates exquisitely against other cyclic dinucleotides, such as c-di-GMP and cyclic-AMP-GMP, via interactions with both the backbone and bases of its cognate second messenger. Small-angle X-ray scattering experiments indicate that global folding of the riboswitch is induced by the two bound cyclic dinucleotides, which bridge the two symmetric three-helix domains. This structural reorganization likely couples c-di-AMP binding to gene expression.
Organizational Affiliation:
Biochemistry and Biophysics Center, National Heart, Lung and Blood Institute, Bethesda, MD, USA.