Small molecule disruptors of the glucokinase-glucokinase regulatory protein interaction: 3. Structure-activity relationships within the aryl carbinol region of the N-arylsulfonamido-N'-arylpiperazine series.
Nishimura, N., Norman, M.H., Liu, L., Yang, K.C., Ashton, K.S., Bartberger, M.D., Chmait, S., Chen, J., Cupples, R., Fotsch, C., Helmering, J., Jordan, S.R., Kunz, R.K., Pennington, L.D., Poon, S.F., Siegmund, A., Sivits, G., Lloyd, D.J., Hale, C., St Jean, D.J.(2014) J Med Chem 57: 3094-3116
- PubMed: 24611879
- DOI: https://doi.org/10.1021/jm5000497
- Primary Citation of Related Structures:
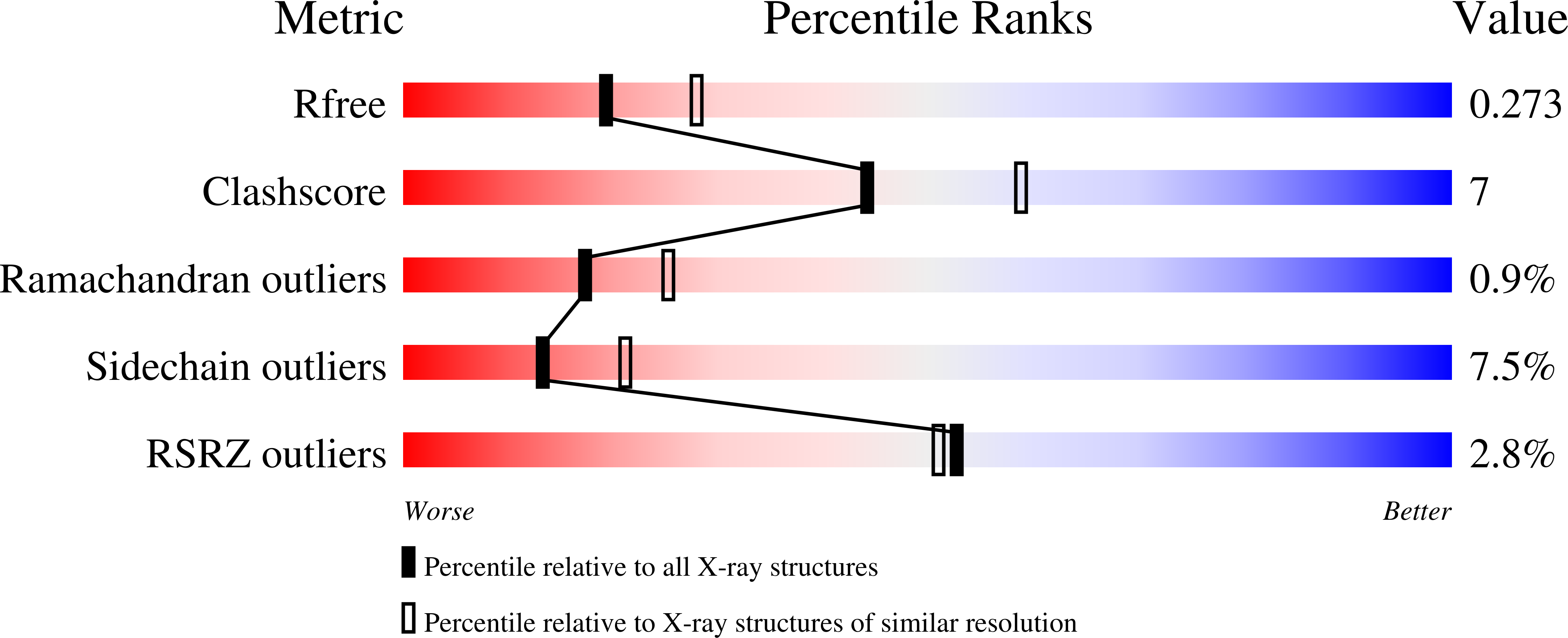
4OHK, 4OHM, 4OHO, 4OHP - PubMed Abstract:
We have recently reported a novel approach to increase cytosolic glucokinase (GK) levels through the binding of a small molecule to its endogenous inhibitor, glucokinase regulatory protein (GKRP). These initial investigations culminated in the identification of 2-(4-((2S)-4-((6-amino-3-pyridinyl)sulfonyl)-2-(1-propyn-1-yl)-1-piperazinyl)phenyl)-1,1,1,3,3,3-hexafluoro-2-propanol (1, AMG-3969), a compound that effectively enhanced GK translocation and reduced blood glucose levels in diabetic animals. Herein we report the results of our expanded SAR investigations that focused on modifications to the aryl carbinol group of this series. Guided by the X-ray cocrystal structure of compound 1 bound to hGKRP, we identified several potent GK-GKRP disruptors bearing a diverse set of functionalities in the aryl carbinol region. Among them, sulfoximine and pyridinyl derivatives 24 and 29 possessed excellent potency as well as favorable PK properties. When dosed orally in db/db mice, both compounds significantly lowered fed blood glucose levels (up to 58%).
Organizational Affiliation:
Department of Therapeutic Discovery-Medicinal Chemistry, ‡Department of Therapeutic Discovery-Molecular Structure and Characterization, §Department of Metabolic Disorders, and ∥Department of Pharmacokinetics and Drug Metabolism, Amgen Inc. , One Amgen Center Drive, Thousand Oaks, California 91320-1799, United States.