Cyclic dinucleotides bind the C-linker of HCN4 to control channel cAMP responsiveness.
Lolicato, M., Bucchi, A., Arrigoni, C., Zucca, S., Nardini, M., Schroeder, I., Simmons, K., Aquila, M., DiFrancesco, D., Bolognesi, M., Schwede, F., Kashin, D., Fishwick, C.W., Johnson, A.P., Thiel, G., Moroni, A.(2014) Nat Chem Biol 10: 457-462
- PubMed: 24776929
- DOI: https://doi.org/10.1038/nchembio.1521
- Primary Citation of Related Structures:
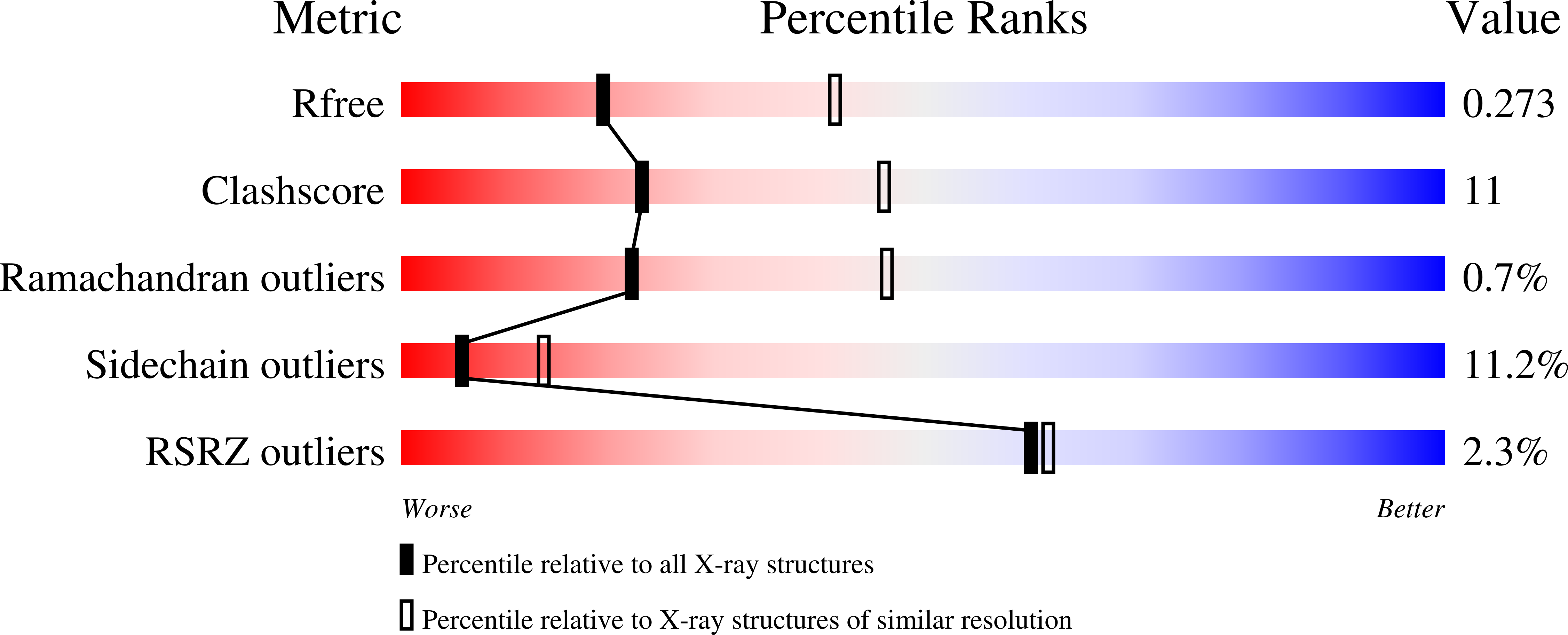
4KL1 - PubMed Abstract:
cAMP mediates autonomic regulation of heart rate by means of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, which underlie the pacemaker current If. cAMP binding to the C-terminal cyclic nucleotide binding domain enhances HCN open probability through a conformational change that reaches the pore via the C-linker. Using structural and functional analysis, we identified a binding pocket in the C-linker of HCN4. Cyclic dinucleotides, an emerging class of second messengers in mammals, bind the C-linker pocket (CLP) and antagonize cAMP regulation of the channel. Accordingly, cyclic dinucleotides prevent cAMP regulation of If in sinoatrial node myocytes, reducing heart rate by 30%. Occupancy of the CLP hence constitutes an efficient mechanism to hinder β-adrenergic stimulation on If. Our results highlight the regulative role of the C-linker and identify a potential drug target in HCN4. Furthermore, these data extend the signaling scope of cyclic dinucleotides in mammals beyond their first reported role in innate immune system.
Organizational Affiliation:
1] Department of Biosciences, University of Milan, Milan, Italy. [2].