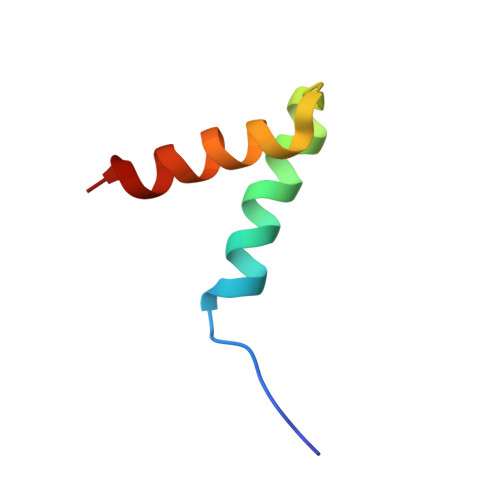
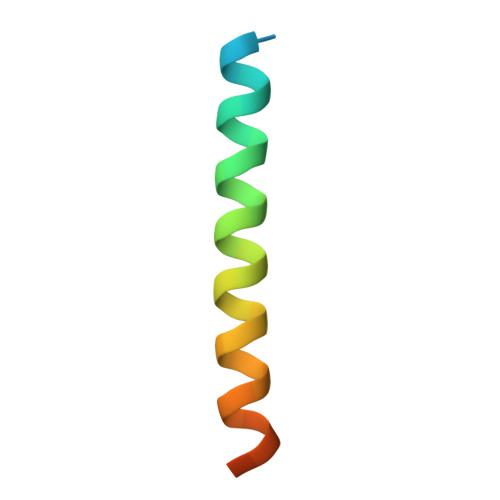
AKAP18:PKA-RII alpha structure reveals crucial anchor points for recognition of regulatory subunits of PKA.
Gotz, F., Roske, Y., Schulz, M.S., Autenrieth, K., Bertinetti, D., Faelber, K., Zuhlke, K., Kreuchwig, A., Kennedy, E.J., Krause, G., Daumke, O., Herberg, F.W., Heinemann, U., Klussmann, E.(2016) Biochem J 473: 1881-1894
- PubMed: 27102985
- DOI: https://doi.org/10.1042/BCJ20160242
- Primary Citation of Related Structures:
4ZP3 - PubMed Abstract:
A-kinase anchoring proteins (AKAPs) interact with the dimerization/docking (D/D) domains of regulatory subunits of the ubiquitous protein kinase A (PKA). AKAPs tether PKA to defined cellular compartments establishing distinct pools to increase the specificity of PKA signalling. Here, we elucidated the structure of an extended PKA-binding domain of AKAP18β bound to the D/D domain of the regulatory RIIα subunits of PKA. We identified three hydrophilic anchor points in AKAP18β outside the core PKA-binding domain, which mediate contacts with the D/D domain. Such anchor points are conserved within AKAPs that bind regulatory RII subunits of PKA. We derived a different set of anchor points in AKAPs binding regulatory RI subunits of PKA. In vitro and cell-based experiments confirm the relevance of these sites for the interaction of RII subunits with AKAP18 and of RI subunits with the RI-specific smAKAP. Thus we report a novel mechanism governing interactions of AKAPs with PKA. The sequence specificity of each AKAP around the anchor points and the requirement of these points for the tight binding of PKA allow the development of selective inhibitors to unequivocally ascribe cellular functions to the AKAP18-PKA and other AKAP-PKA interactions.
Organizational Affiliation:
Max Delbrück Centre for Molecular Medicine Berlin in the Helmholtz Association (MDC), Robert-Rössle-Straße 10, 13125 Berlin, Germany Leibniz Institute for Molecular Pharmacology (FMP), Robert-Rössle-Straße 10, 13125 Berlin, Germany.