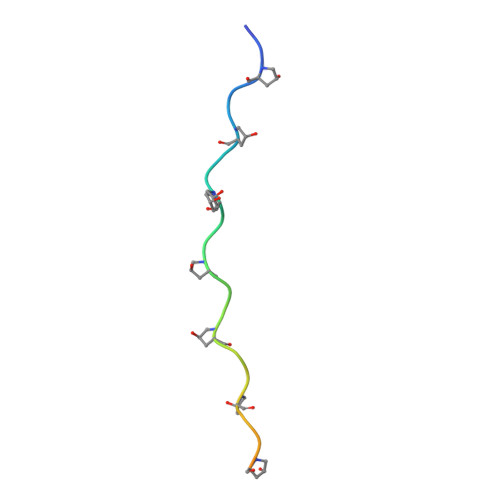
Sub-angstrom structure of the collagen model peptide (GPO)10 shows a hydrated triple helix with pitch variation and two proline ring conformations
Suzuki, H., Mahapatra, D., Steel, P.J., Dyer, J., Dobson, R.C.J., Gerrard, J.A., Valery, C.To be published.