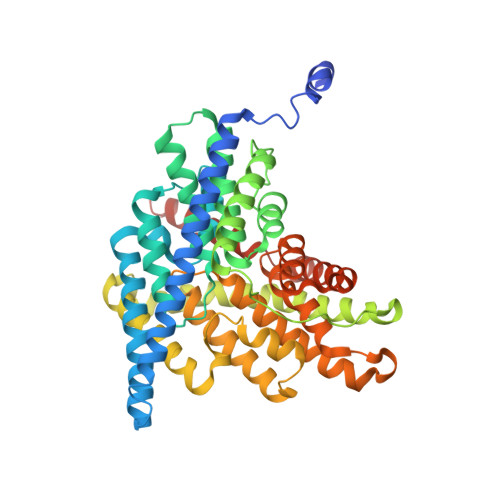
Intracellular proton-transfer mutants in a CLC Cl-/H+ exchanger.
Lim, H.H., Miller, C.(2009) J Gen Physiol 133: 131-138
- PubMed: 19139174
- DOI: https://doi.org/10.1085/jgp.200810112
- Primary Citation of Related Structures:
3EJY, 3EJZ - PubMed Abstract:
CLC-ec1, a bacterial homologue of the CLC family's transporter subclass, catalyzes transmembrane exchange of Cl(-) and H(+). Mutational analysis based on the known structure reveals several key residues required for coupling H(+) to the stoichiometric countermovement of Cl(-). E148 (Glu(ex)) transfers protons between extracellular water and the protein interior, and E203 (Glu(in)) is thought to function analogously on the intracellular face of the protein. Mutation of either residue eliminates H(+) transport while preserving Cl(-) transport. We tested the role of Glu(in) by examining structural and functional properties of mutants at this position. Certain dissociable side chains (E, D, H, K, R, but not C and Y) retain H(+)/Cl(-) exchanger activity to varying degrees, while other mutations (V, I, or C) abolish H(+) coupling and severely inhibit Cl(-) flux. Transporters substituted with other nonprotonatable side chains (Q, S, and A) show highly impaired H(+) transport with substantial Cl(-) transport. Influence on H(+) transport of side chain length and acidity was assessed using a single-cysteine mutant to introduce non-natural side chains. Crystal structures of both coupled (E203H) and uncoupled (E203V) mutants are similar to wild type. The results support the idea that Glu(in) is the internal proton-transfer residue that delivers protons from intracellular solution to the protein interior, where they couple to Cl(-) movements to bring about Cl(-)/H(+) exchange.
Organizational Affiliation:
Department of Biochemistry, Howard Hughes Medical Institute, Brandeis University, Waltham, MA 02454, USA.