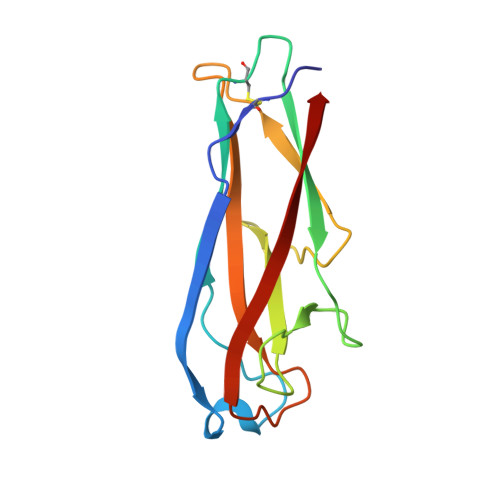
Infinite Kinetic Stability against Dissociation of Supramolecular Protein Complexes through Donor Strand Complementation
Puorger, C., Eidam, O., Capitani, G., Erilov, D., Grutter, M.G., Glockshuber, R.(2008) Structure 16: 631-642
- PubMed: 18400183
- DOI: https://doi.org/10.1016/j.str.2008.01.013
- Primary Citation of Related Structures:
3BFQ, 3BFW - PubMed Abstract:
Adhesive type 1 pili from uropathogenic Escherichia coli strains are heat and denaturant resistant, filamentous protein complexes. Individual pilus subunits associate through "donor strand complementation," whereby the incomplete immunoglobulin-like fold of each subunit is completed by the N-terminal extension of a neighboring subunit. We show that antiparallel donor strand insertion generally causes nonequilibrium behavior in protein folding and extreme activation energy barriers for dissociation of subunit-subunit complexes. We identify the most kinetically stable, noncovalent protein complex known to date. The complex between the pilus subunit FimG and the donor strand peptide of the subunit FimF shows an extrapolated dissociation half-life of 3 x 10(9) years. The 15 residue peptide forms ideal intermolecular beta sheet H-bonds with FimG over 10 residues, and its hydrophobic side chains strongly interact with the hydrophobic core of FimG. The results show that kinetic stability and nonequilibrium behavior in protein folding confers infinite stability against dissociation in extracellular protein complexes.
Organizational Affiliation:
ETH Zürich, Institute of Molecular Biology and Biophysics, CH-8093 Zurich, Switzerland.