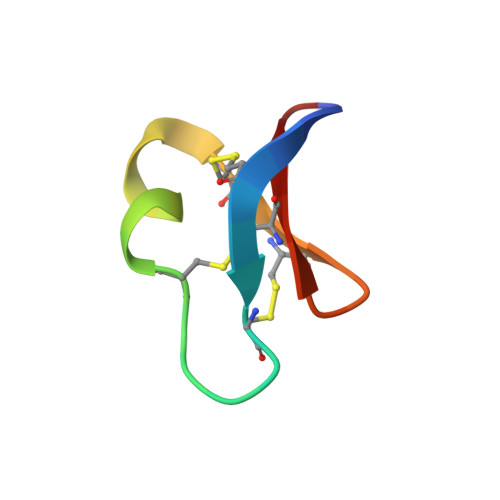
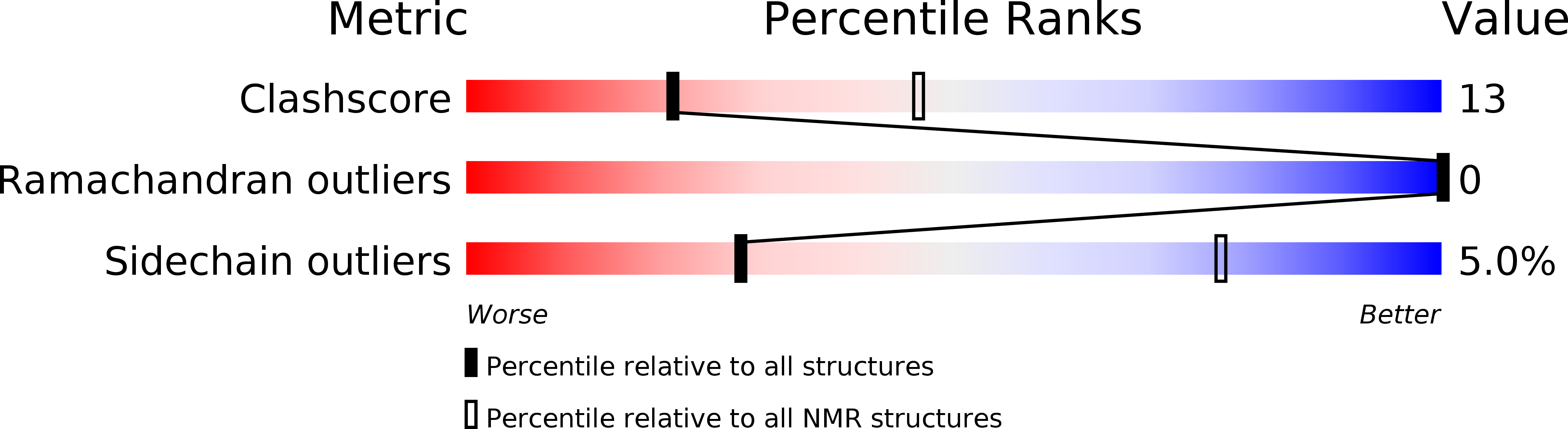Structural and biochemical characteristics of the cyclotide kalata B5 from Oldenlandia affinis
Plan, M.R., Rosengren, K.J., Sando, L., Daly, N.L., Craik, D.J.(2010) Biopolymers 94: 647-658
- PubMed: 20564013
- DOI: https://doi.org/10.1002/bip.21409
- Primary Citation of Related Structures:
2KUX - PubMed Abstract:
Cyclotides are a large family of plant-derived proteins typified by their head-to-tail cyclic backbone and knotted arrangement of three disulfide bonds. Although they display a diverse range of biological activities, their native function is thought to be plant defense. Here we characterized the expression, three-dimensional structure, and hemolytic activity of the cyclotide kalata B5 from the African plant Oldenlandia affinis. Kalata B5 shows an interesting seasonal variation in its expression and can only be isolated during certain times of the year, when the plant is flowering. It displays a typical tightly folded cyclic Scystine knot structure. A range of pH and temperature titrations reveal that a conserved glutamic acid in loop 1 Sof the structure forms a key hydrogen bond network, similar to that reported previously for other cyclotides. However, specific line broadening in the NMR spectra of kalata B5 suggests that the hydrogen bonding network in this peptide is less rigid than in other cyclotides. Notably, the pK9a) of Glu6 of 4.5 is higher than the values for other cyclotides studied so far, which range from 3.0 to 4.0, providing a further indication of a weaker hydrogen bond network. Kalata B5 has only moderate hemolytic activity compared with other highly expressed cyclotides, and this reduced activity probably reflects its more flexible structure. As is the case with other cyclotides, kalata B5 has an exposed hydrophobic region on its surface, supporting suggestions that this hydrophobic patch is a key feature for membrane binding and biological activity of cyclotides.
Organizational Affiliation:
Institute for Molecular Bioscience, Division of Chemistry and Structural Biology, The University of Queensland, 306 Carmody Rd, St. Lucia, QLD 4067, Australia.