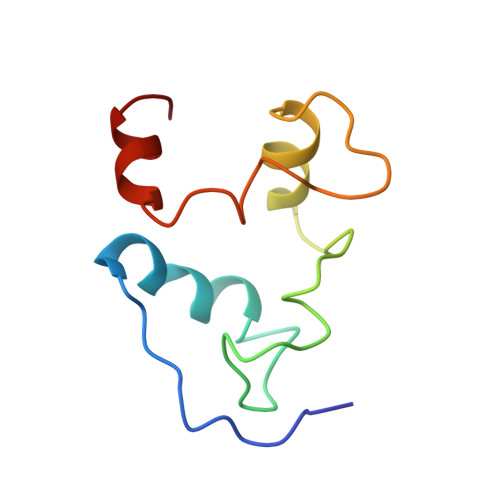
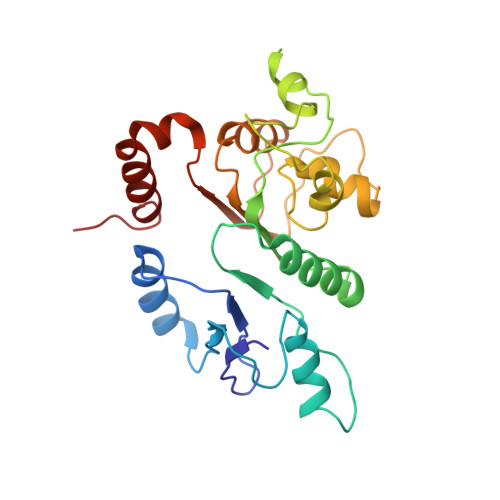
Structural basis for the selectivity of the external thioesterase of the surfactin synthetase.
Koglin, A., Lohr, F., Bernhard, F., Rogov, V.V., Frueh, D.P., Strieter, E.R., Mofid, M.R., Guntert, P., Wagner, G., Walsh, C.T., Marahiel, M.A., Dotsch, V.(2008) Nature 454: 907-911
- PubMed: 18704089
- DOI: https://doi.org/10.1038/nature07161
- Primary Citation of Related Structures:
2K2Q, 2RON - PubMed Abstract:
Non-ribosomal peptide synthetases (NRPS) and polyketide synthases (PKS) found in bacteria, fungi and plants use two different types of thioesterases for the production of highly active biological compounds. Type I thioesterases (TEI) catalyse the release step from the assembly line of the final product where it is transported from one reaction centre to the next as a thioester linked to a 4'-phosphopantetheine (4'-PP) cofactor that is covalently attached to thiolation (T) domains. The second enzyme involved in the synthesis of these secondary metabolites, the type II thioesterase (TEII), is a crucial repair enzyme for the regeneration of functional 4'-PP cofactors of holo-T domains of NRPS and PKS systems. Mispriming of 4'-PP cofactors by acetyl- and short-chain acyl-residues interrupts the biosynthetic system. This repair reaction is very important, because roughly 80% of CoA, the precursor of the 4'-PP cofactor, is acetylated in bacteria. Here we report the three-dimensional structure of a type II thioesterase from Bacillus subtilis free and in complex with a T domain. Comparison with structures of TEI enzymes shows the basis for substrate selectivity and the different modes of interaction of TEII and TEI enzymes with T domains. Furthermore, we show that the TEII enzyme exists in several conformations of which only one is selected on interaction with its native substrate, a modified holo-T domain.
Organizational Affiliation:
Institute of Biophysical Chemistry and Center for Biomolecular Magnetic Resonance and Cluster of Excellence Macromolecular Complexes (CEF), J.W.-Goethe University, 60438 Frankfurt am Main, Germany.