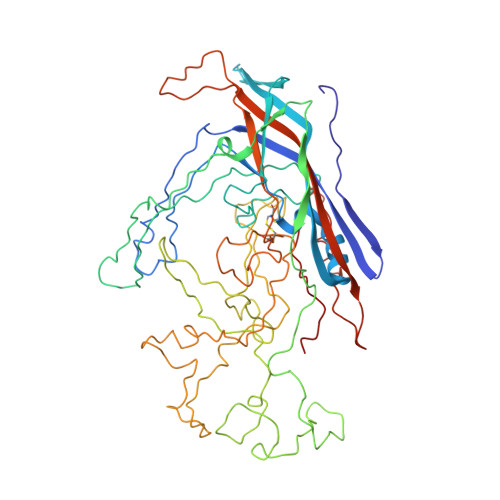
The canine parvovirus empty capsid structure.
Wu, H., Rossmann, M.G.(1993) J Mol Biol 233: 231-244
- PubMed: 8377200
- DOI: https://doi.org/10.1006/jmbi.1993.1502
- Primary Citation of Related Structures:
2CAS - PubMed Abstract:
The structure of empty canine parvovirus capsids shows that residues 37 to the carboxy-terminal residue 584 (VP2 numbering) are ordered in each of the 60 subunits. The central structural motif of each subunit is the eight-stranded antiparallel beta-barrel that has been found in many other virus structures. Five beta-hairpin turns form a beta-cylindrical structure at each icosahedral 5-fold axis. The N-terminal glycine-rich sequence can be accommodated within this cylinder without excessive steric hindrance, consistent with the electron density distribution. By far the largest conformational differences between the full and empty virus were found in the region where some ordered DNA has been observed to bind in canine parvovirus full particles. Extensive interactions among 3-fold related subunits indicate that a trimeric subunit might be a viral assembly intermediate.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, West Lafayette, IN 47907.