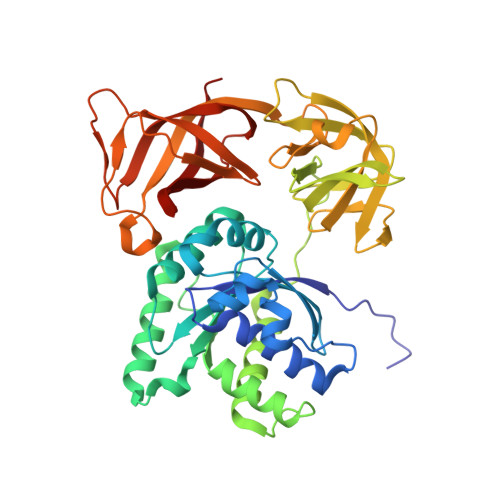
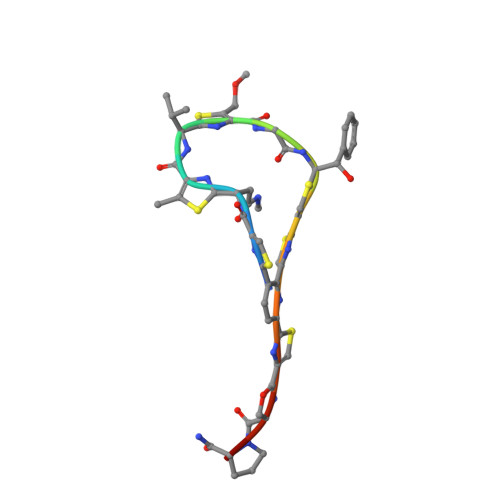
Structural basis of the action of pulvomycin and GE2270 A on elongation factor Tu.
Parmeggiani, A., Krab, I.M., Okamura, S., Nielsen, R.C., Nyborg, J., Nissen, P.(2006) Biochemistry 45: 6846-6857
- PubMed: 16734421
- DOI: https://doi.org/10.1021/bi0525122
- Primary Citation of Related Structures:
2C77, 2C78 - PubMed Abstract:
Pulvomycin inhibits protein synthesis by preventing the formation of the ternary complex between elongation factor Tu (EF-Tu) x GTP and aa-tRNA. In this work, the crystal structure of Thermus thermophilus EF-Tu x pulvomycin in complex with the GTP analogue guanylyl imino diphosphate (GDPNP) at 1.4 A resolution reveals an antibiotic binding site extending from the domain 1-3 interface to domain 2, overlapping the domain 1-2-3 junction. Pulvomycin binding interferes with the binding of the 3'-aminoacyl group, the acceptor stem, and 5' end of tRNA. Only part of pulvomycin overlaps the binding site of GE2270 A, a domain 2-bound antibiotic of a structure unrelated to pulvomycin, which also hinders aa-tRNA binding. The structure of the T. thermophilus EF-Tu x GDPNP x GE2270 A complex at 1.6 A resolution shows that GE2270 A interferes with the binding of the 3'-aminoacyl group and part of the acceptor stem of aa-tRNA but not with the 5' end. Both compounds, pulvomycin more markedly, hinder the correct positioning of domain 1 over domains 2 and 3 that characterizes the active form of EF-Tu, while they affect the domain 1 switch regions that control the EF-Tu x GDP/GTP transitions in different ways. This work reveals how two antibiotics with different structures and binding modes can employ a similar mechanism of action.
Organizational Affiliation:
Department of Molecular Biology, University of Aarhus, Gustav Wieds Vej 10 C, DK-8000 Aarhus C, Denmark. andrea@bioxray.dk