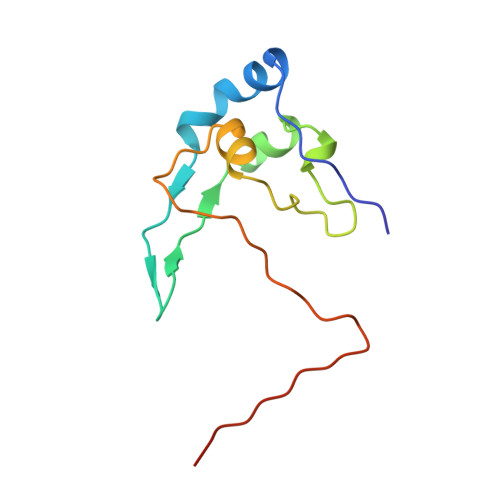
Crystal structure of the yeast cell-cycle control protein, p13suc1, in a strand-exchanged dimer.
Khazanovich, N., Bateman, K., Chernaia, M., Michalak, M., James, M.(1996) Structure 4: 299-309
- PubMed: 8805536
- DOI: https://doi.org/10.1016/s0969-2126(96)00034-2
- Primary Citation of Related Structures:
1PUC - PubMed Abstract:
p13(suc1) from fission yeast is a member of the CDC28 kinase specific (CKS) class of cell-cycle control proteins, that includes CKS1 from budding yeast and the human homologues CksHs1 and CksHs2. p13(suc1) participates in the regulation of p34(cdc2), a cyclin-dependent kinase controlling the G1-S and the G2-M transitions of the cell cycle. The CKS proteins are believed to exert their regulatory activity by binding to the kinase, in which case their function may be governed by their conformation or oligomerization state. Previously determined X-ray structures of p13(suc1), CksHs1 and CksHs2 show that these proteins share a common fold but adopt different oligomeric states. Monomeric forms of p13(suc1) and CksHs1 have been solved. In addition, CksHs2 and p13(suc1) have been observed by X-ray crystallography in assemblies of strand-exchanged dimers. Analysis of various assemblies of the CKS proteins, as found in different crystal forms, should help to clarify their role in cell-cycle control.
Organizational Affiliation:
Medical Research Council Group in Protein Structure and Function, University of Alberta, Edmonton, Alberta, Canada T6G 2H7.