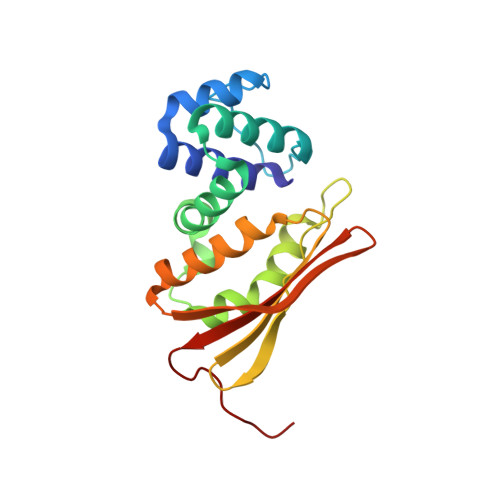
Crystal structure of an oxygen-binding heme domain related to soluble guanylate cyclases.
Pellicena, P., Karow, D.S., Boon, E.M., Marletta, M.A., Kuriyan, J.(2004) Proc Natl Acad Sci U S A 101: 12854-12859
- PubMed: 15326296
- DOI: https://doi.org/10.1073/pnas.0405188101
- Primary Citation of Related Structures:
1U4H, 1U55, 1U56 - PubMed Abstract:
Soluble guanylate cyclases are nitric oxide-responsive signaling proteins in which the nitric oxide sensor is a heme-binding domain of unknown structure that we have termed the heme-NO and oxygen binding (H-NOX) domain. H-NOX domains are also found in bacteria, either as isolated domains, or are fused through a membrane-spanning region to methyl-accepting chemotaxis proteins. We have determined the crystal structure of an oxygen-binding H-NOX domain of one such signaling protein from the obligate anaerobe Thermoanaerobacter tengcongensis at 1.77-angstroms resolution, revealing a protein fold unrelated to known structures. Particularly striking is the structure of the protoporphyrin IX group, which is distorted from planarity to an extent not seen before in protein-bound heme groups. Comparison of the structure of the H-NOX domain in two different crystal forms suggests a mechanism whereby alteration in the degree of distortion of the heme group is coupled to changes on the molecular surface of the H-NOX domain and potentially to changes in intermolecular interactions.
Organizational Affiliation:
Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720, USA.