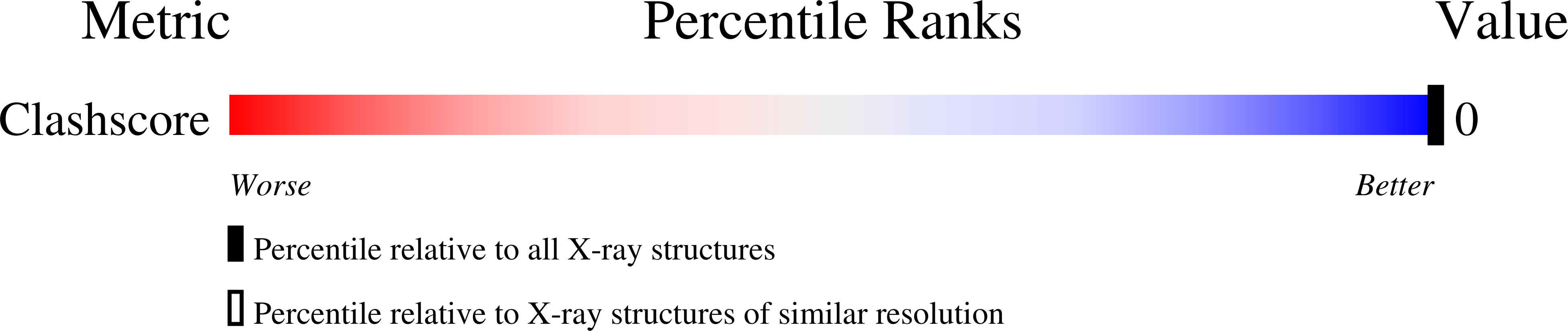
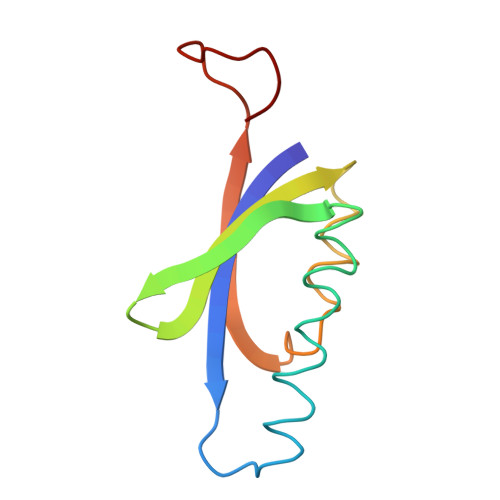
Crystal structure of muconolactone isomerase at 3.3 A resolution.
Katti, S.K., Katz, B.A., Wyckoff, H.W.(1989) J Mol Biol 205: 557-571
- PubMed: 2926818
- DOI: https://doi.org/10.1016/0022-2836(89)90226-x
- Primary Citation of Related Structures:
1MLI - PubMed Abstract:
The crystal structure of muconolactone isomerase from Pseudomonas putida, a unique molecule with ten 96 amino acid subunits and 5-fold, and 2-fold symmetries, has been solved at 3.3 A resolution. The non-crystallographic symmetries were used to refine the initial single isomorphous replacement phases and produce an interpretable 10-fold averaged map. The backbone trace is complete and confirmed by the amino acid sequence fit. Each subunit is composed of a body with two alpha-helices and an antiparallel twisted beta-sheet of four strands, and an extended arm. The helices and the sheet fold to form a two-layered structure with an enclosed hydrophobic core and a partially formed putative active site pocket. The C-terminal arm of another subunit related by a local dyad symmetry extends over the core to complete this pocket. The decameric protein is almost spherical, with the helices forming the external coat. There is a large hydrophilic cavity in the center with open ends along the 5-fold axis. Molecular interactions between subunits are extensive. Each subunit contacts four neighbors and loses nearly 40% of its solvent contact area on oligomerization.
Organizational Affiliation:
Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06511.