A possible physiological function and the tertiary structure of a 4-kDa peptide in legumes
Yamazaki, T., Takaoka, M., Katoh, E., Hanada, K., Sakita, M., Sakata, K., Nishiuchi, Y., Hirano, H.(2003) Eur J Biochem 270: 1269-1276
- PubMed: 12631285
- DOI: https://doi.org/10.1046/j.1432-1033.2003.03489.x
- Primary Citation of Related Structures:
1JU8 - PubMed Abstract:
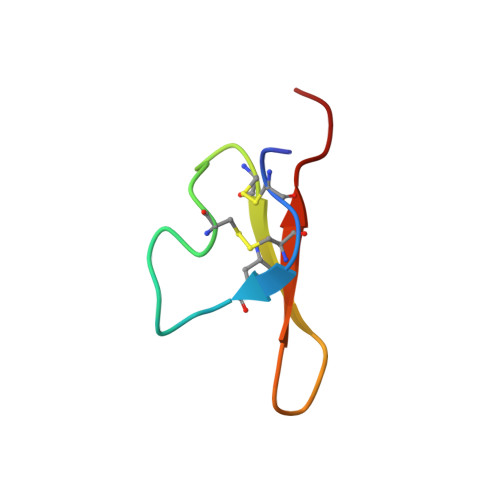
Previously, we isolated a 4-kDa peptide capable of binding to a 43-kDa receptor-like protein and stimulating protein kinase activity of the 43-kDa protein in soybean. Both of them were found to localize in the plasma membranes and cell walls. Here, we report the physiological effects of 4-kDa peptide expressed transiently in the cultured carrot and bird's-foot trefoil cells transfected with pBI 121 plasmid containing the 4-kDa peptide gene. At early developmental stage, the transgenic callus grew rapidly compared to the wild callus in both species. Cell proliferation of in vitro cultured nonembryogenic carrot callus was apparently affected with the 4-kDa peptide in the medium. Complementary DNAs encoding the 4-kDa peptide from mung bean and azuki bean were cloned by PCR and sequenced. The amino-acid sequences deduced from the nucleotide sequences are homologous among legume species, particularly, the sites of cysteine residues are highly conserved. This conserved sequence reflects the importance of intradisulfide bonds required for the 4-kDa peptide to perform its function. Three dimensional structure of the 4-kDa peptide determined by NMR spectroscopy suggests that this peptide is a T-knot scaffold containing three beta-strands, and the specific binding activity to the 43-kDa protein and stimulatory effect on the protein phosphorylation could be attributed to the spatial arrangements of hydrophobic residues at the solvent-exposed surface of two-stranded beta-sheet of 4-kDa peptide. The importance of these residues for the 4-kDa peptide to bind to the 43-kDa protein was indicated by site-directed mutagenesis. These results suggest that the 4-kDa peptide is a hormone-like peptide and the 43-kDa protein is involved in cellular signal transduction of the peptide.
Organizational Affiliation:
National Institute of Agrobiological Sciences, Tsukuba, Ibaraki, Japan.