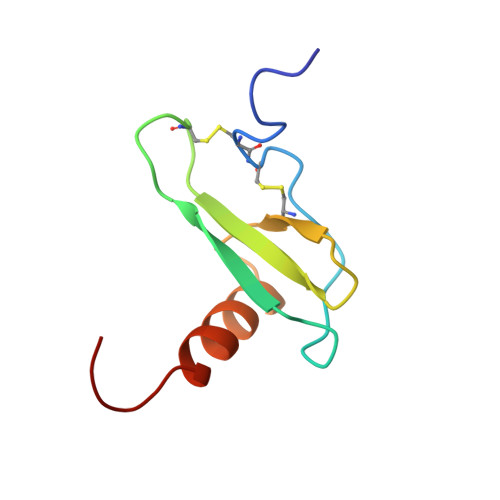
Solution structure of eotaxin, a chemokine that selectively recruits eosinophils in allergic inflammation.
Crump, M.P., Rajarathnam, K., Kim, K.S., Clark-Lewis, I., Sykes, B.D.(1998) J Biol Chem 273: 22471-22479
- PubMed: 9712872
- DOI: https://doi.org/10.1074/jbc.273.35.22471
- Primary Citation of Related Structures:
1EOT, 2EOT - PubMed Abstract:
The solution structure of the CCR3-specific chemokine, eotaxin, has been determined by NMR spectroscopy. The quaternary structure of eotaxin was investigated by ultracentrifugation and NMR, and it was found to be in equilibrium between monomer and dimer under a wide range of conditions. At pH
Organizational Affiliation:
Protein Engineering Network of Centres of Excellence (PENCE) and Department of Biochemistry, University of Alberta, Edmonton, Alberta, Canada T6G 2S2.