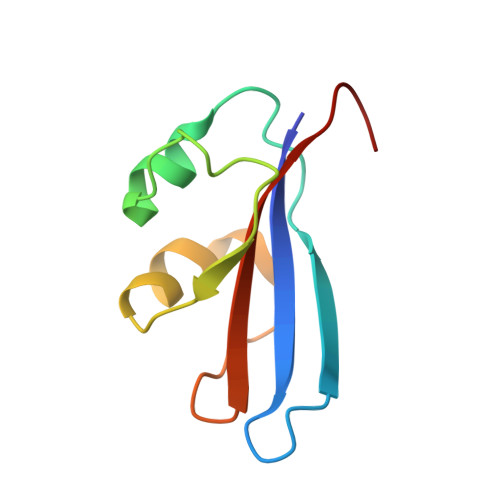
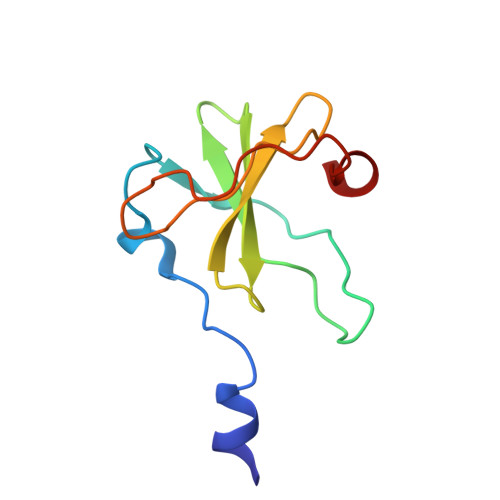
Inhibition of a Ribosome Inactivating Ribonuclease: The Crystal Structure of the Cytotoxic Domain of Colicin E3 in Complex with its Immunity Protein
Carr, S., Walker, D., James, R., Kleanthous, C., Hemmings, A.M.(2000) Structure 8: 949
- PubMed: 10986462
- DOI: https://doi.org/10.1016/s0969-2126(00)00186-6
- Primary Citation of Related Structures:
1E44 - PubMed Abstract:
The cytotoxicity of most ribonuclease E colicins towards Escherichia coli arises from their ability to specifically cleave between bases 1493 and 1494 of 16S ribosomal RNA. This activity is carried by the C-terminal domain of the colicin, an activity which if left unneutralised would lead to destruction of the producing cell. To combat this the host E. coli cell produces an inhibitor protein, the immunity protein, which forms a complex with the ribonuclease domain effectively suppressing its activity.
Organizational Affiliation:
Colicin Research Group, School of Chemical Sciences, University of East Anglia, NR4 7TJ, Norwich, UK.