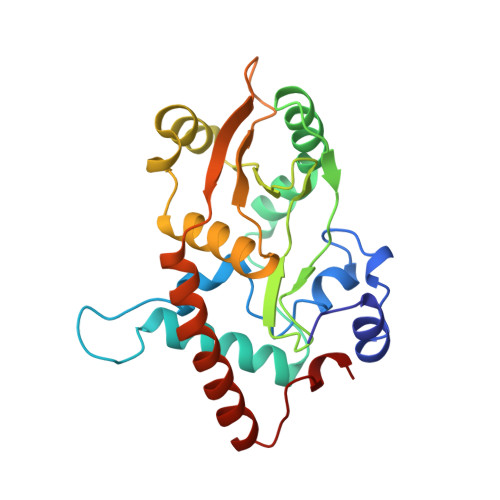
Toroidal structure of lambda-exonuclease.
Kovall, R., Matthews, B.W.(1997) Science 277: 1824-1827
- PubMed: 9295273
- DOI: https://doi.org/10.1126/science.277.5333.1824
- Primary Citation of Related Structures:
1AVQ - PubMed Abstract:
Structure determination at 2.4 angstrom resolution shows that lambda-exonuclease consists of three subunits that form a toroid. The central channel is funnel shaped, tapering from an inner diameter of about 30 angstroms at the wider end to 15 angstroms at the narrow end. This is adequate to accommodate the DNA substrate and thus provides a structural basis for the ability of the enzyme to sequentially hydrolyze thousands of nucleotides in a highly processive manner. The results also suggest the locations of the active sites and the constraints that limit cleavage to a single strand.
Organizational Affiliation:
Institute of Molecular Biology, Howard Hughes Medical Institute, and Department of Physics, University of Oregon, Eugene, OR 97403, USA.