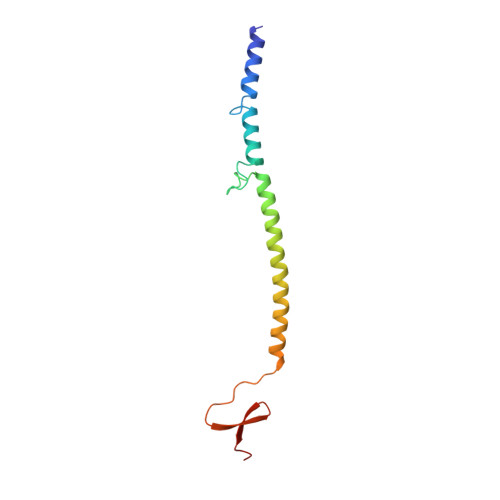
Structure of bacteriophage T4 fibritin: a segmented coiled coil and the role of the C-terminal domain.
Tao, Y., Strelkov, S.V., Mesyanzhinov, V.V., Rossmann, M.G.(1997) Structure 5: 789-798
- PubMed: 9261070
- DOI: https://doi.org/10.1016/s0969-2126(97)00233-5
- Primary Citation of Related Structures:
1AA0 - PubMed Abstract:
Oligomeric coiled-coil motifs are found in numerous protein structures; among them is fibritin, a structural protein of bacteriophage T4, which belongs to a class of chaperones that catalyze a specific phage-assembly process. Fibritin promotes the assembly of the long tail fibers and their subsequent attachment to the tail baseplate; it is also a sensing device that controls the retraction of the long tail fibers in adverse environments and, thus, prevents infection. The structure of fibritin had been predicted from sequence and biochemical analyses to be mainly a triple-helical coiled coil. The determination of its structure at atomic resolution was expected to give insights into the assembly process and biological function of fibritin, and the properties of modified coiled-coil structures in general.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, West Lafayette, Indiana 47907-1392, USA.