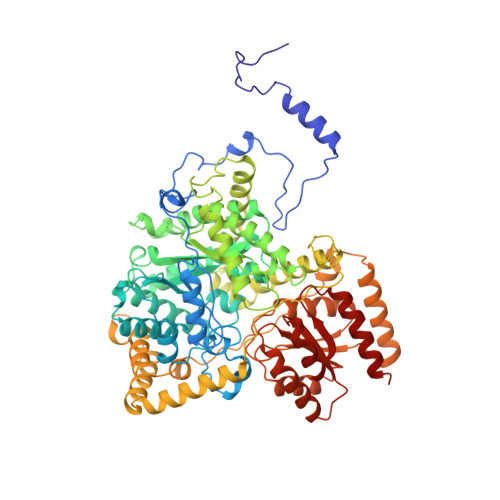
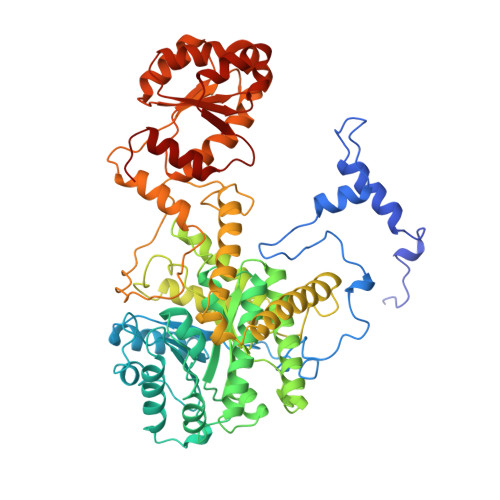
Crystal structure of substrate complexes of methylmalonyl-CoA mutase.
Mancia, F., Smith, G.A., Evans, P.R.(1999) Biochemistry 38: 7999-8005
- PubMed: 10387043
- DOI: https://doi.org/10.1021/bi9903852
- Primary Citation of Related Structures:
6REQ, 7REQ - PubMed Abstract:
X-ray crystal structures of methylmalonyl-CoA mutase in complexes with substrate methylmalonyl-CoA and inhibitors 2-carboxypropyl-CoA and 3-carboxypropyl-CoA (substrate and product analogues) show that the enzyme-substrate interactions change little during the course of the rearrangement reaction, in contrast to the large conformational change on substrate binding. The substrate complex shows a 5'-deoxyadenine molecule in the active site, bound weakly and not attached to the cobalt atom of coenzyme B12, rotated and shifted from its position in the substrate-free adenosylcobalamin complex. The position of Tyralpha89 close to the substrate explains the stereochemical selectivity of the enzyme for (2R)-methylmalonyl-CoA.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Cambridge, UK.