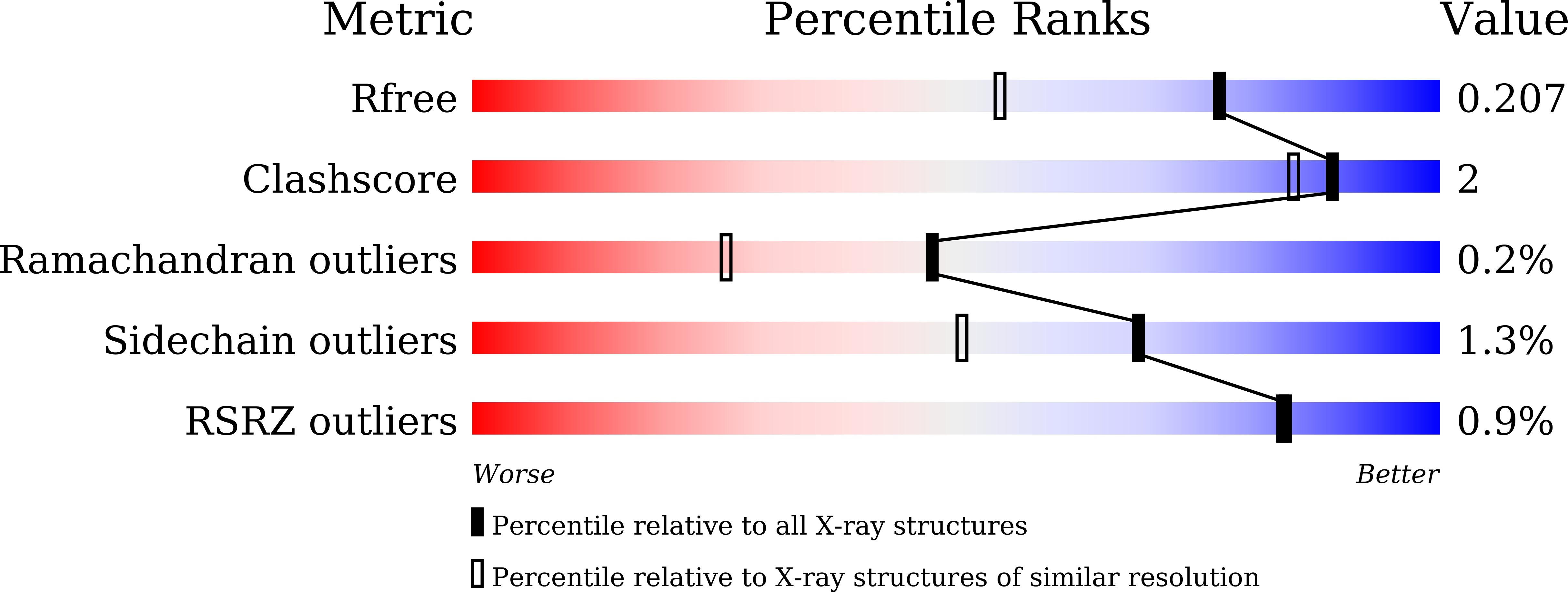
Characterization and structural analyses of a novel glycosyltransferase acting on the beta-1,2-glucosidic linkages.
Kobayashi, K., Shimizu, H., Tanaka, N., Kuramochi, K., Nakai, H., Nakajima, M., Taguchi, H.(2022) J Biol Chem 298: 101606-101606
- PubMed: 35065074
- DOI: https://doi.org/10.1016/j.jbc.2022.101606
- Primary Citation of Related Structures:
7VKW, 7VKX, 7VKY, 7VKZ, 7VL0, 7VL1, 7VL2, 7VL3, 7VL4, 7VL5, 7VL6, 7VL7, 7X87 - PubMed Abstract:
The IALB_1185 protein, which is encoded in the gene cluster for endo-β-1,2-glucanase homologs in the genome of Ignavibacterium album, is a glycoside hydrolase family (GH) 35 protein. However, most known GH35 enzymes are β-galactosidases, which is inconsistent with the components of this gene cluster. Thus, IALB_1185 is expected to possess novel enzymatic properties. Here, we showed using recombinant IALB_1185 that this protein has glycosyltransferase activity toward β-1,2-glucooligosaccharides, and that the kinetic parameters for β-1,2-glucooligosaccharides are not within the ranges for general GH enzymes. When various aryl- and alkyl-glucosides were used as acceptors, glycosyltransfer products derived from these acceptors were subsequently detected. Kinetic analysis further revealed that the enzyme has wide aglycone specificity regardless of the anomer, and that the β-1,2-linked glucose dimer sophorose is an appropriate donor. In the complex of wild-type IALB_1185 with sophorose, the electron density of sophorose was clearly observed at subsites -1 and +1, whereas in the E343Q mutant-sophorose complex, the electron density of sophorose was clearly observed at subsites +1 and +2. This observation suggests that binding at subsites -1 and +2 competes through Glu102, which is consistent with the preference for sophorose as a donor and unsuitability of β-1,2-glucooligosaccharides as acceptors. A pliable hydrophobic pocket that can accommodate various aglycone moieties was also observed in the complex structures with various glucosides. Overall, our biochemical and structural data are indicative of a novel enzymatic reaction. We propose that IALB_1185 be redefined β-1,2-glucooligosaccharide:d-glucoside β-d-glucosyltransferase as a systematic name and β-1,2-glucosyltransferase as an accepted name.
Organizational Affiliation:
The Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science, Chiba, Japan.