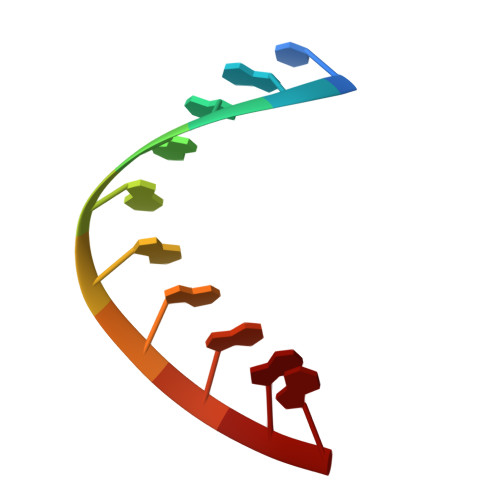
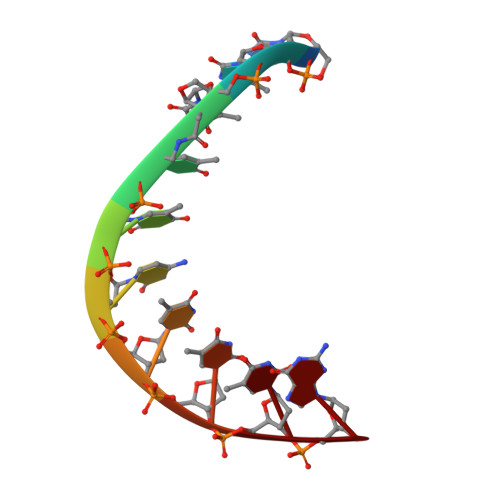
An LNA-amide modification that enhances the cell uptake and activity of phosphorothioate exon-skipping oligonucleotides.
Baker, Y.R., Thorpe, C., Chen, J., Poller, L.M., Cox, L., Kumar, P., Lim, W.F., Lie, L., McClorey, G., Epple, S., Singleton, D., McDonough, M.A., Hardwick, J.S., Christensen, K.E., Wood, M.J.A., Hall, J.P., El-Sagheer, A.H., Brown, T.(2022) Nat Commun 13: 4036-4036
- PubMed: 35821218
- DOI: https://doi.org/10.1038/s41467-022-31636-2
- Primary Citation of Related Structures:
7NRP, 7OOO, 7OOS, 7OZZ - PubMed Abstract:
Oligonucleotides that target mRNA have great promise as therapeutic agents for life-threatening conditions but suffer from poor bioavailability, hence high cost. As currently untreatable diseases come within the reach of oligonucleotide therapies, new analogues are urgently needed to address this. With this in mind we describe reduced-charge oligonucleotides containing artificial LNA-amide linkages with improved gymnotic cell uptake, RNA affinity, stability and potency. To construct such oligonucleotides, five LNA-amide monomers (A, T, C, 5mC and G), where the 3'-OH is replaced by an ethanoic acid group, are synthesised in good yield and used in solid-phase oligonucleotide synthesis to form amide linkages with high efficiency. The artificial backbone causes minimal structural deviation to the DNA:RNA duplex. These studies indicate that splice-switching oligonucleotides containing LNA-amide linkages and phosphorothioates display improved activity relative to oligonucleotides lacking amides, highlighting the therapeutic potential of this technology.
Organizational Affiliation:
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA, UK.