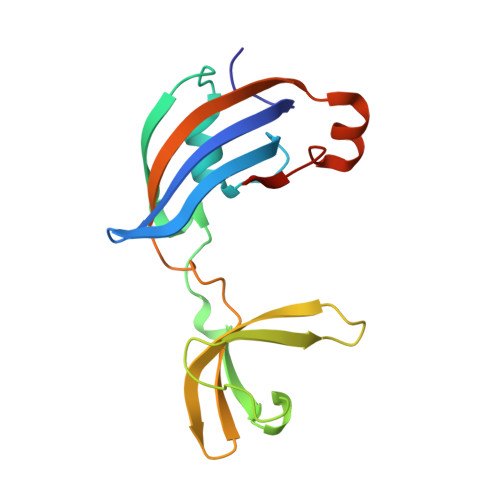
Impact of distant peptide substrate residues on enzymatic activity of SlyD.
Pazicky, S., Werle, A.A., Lei, J., Low, C., Weininger, U.(2022) Cell Mol Life Sci 79: 138-138
- PubMed: 35184231
- DOI: https://doi.org/10.1007/s00018-022-04179-4
- Primary Citation of Related Structures:
7OXG, 7OXH, 7OXI, 7OXJ, 7OXK - PubMed Abstract:
Peptidyl-prolyl isomerases (PPIases) catalyze intrinsically slow and often rate-limiting isomerization of prolyl-peptide bonds in unfolded or partially folded proteins, thereby speeding up the folding process and preventing misfolding. They often possess binding and chaperone domains in addition to the domain carrying the isomerization activity. Although generally, their substrates display no identity in their amino acid sequence upstream and downstream of the proline with 20 possibilities for each residue, PPIases are efficient enzymes. SlyD is a highly efficient PPIase consisting of an isomerase domain and an additional chaperone domain. The binding of peptide substrates to SlyD and its enzymatic activity depend to some extend on the proline-proximal residues, however, the impact of proline-distant residues has not been investigated so far. Here, we introduce a label-free NMR-based method to measure SlyD activity on different peptide substrates and analysed the data in the context of obtained binding affinities and several co-crystal structures. We show that especially charged and aromatic residues up to eight positions downstream and three positions upstream of the proline and outside the canonical region of similar conformations affect the activity and binding, although they rarely display distinct conformations in our crystal structures. We hypothesize that these positions primarily influence the association reaction. In the absence of the chaperone domain the isomerase activity strongly correlates with substrate affinity, whereas additional factors play a role in its presence. The mutual orientation of isomerase and chaperone domains depends on the presence of substrates in both binding sites, implying allosteric regulation of enzymatic activity.
Organizational Affiliation:
Centre for Structural Systems Biology (CSSB), Notkestrasse 85, 22607, Hamburg, Germany.