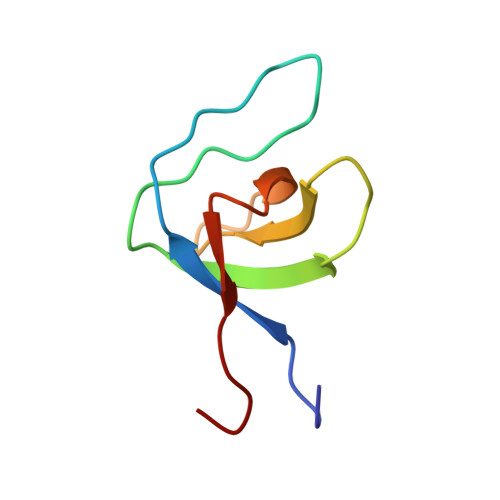
Structure of SNX9 SH3 in complex with a viral ligand reveals the molecular basis of its unique specificity for alanine-containing class I SH3 motifs.
Tossavainen, H., Ugurlu, H., Karjalainen, M., Hellman, M., Antenucci, L., Fagerlund, R., Saksela, K., Permi, P.(2022) Structure 30: 828
- PubMed: 35390274
- DOI: https://doi.org/10.1016/j.str.2022.03.006
- Primary Citation of Related Structures:
7OJ9 - PubMed Abstract:
Class I SH3 domain-binding motifs generally comply with the consensus sequence [R/K]xØPxxP, the hydrophobic residue Ø being proline or leucine. We have studied the unusual Ø = Ala-specificity of SNX9 SH3 by determining its complex structure with a peptide present in eastern equine encephalitis virus (EEEV) nsP3. The structure revealed the length and composition of the n-Src loop as important factors determining specificity. We also compared the affinities of EEEV nsP3 peptide, its mutants, and cellular ligands to SNX9 SH3. These data suggest that nsP3 has evolved to minimize reduction of conformational entropy upon binding, hence acquiring stronger affinity, enabling takeover of SNX9. The RxAPxxP motif was also found in human T cell leukemia virus-1 (HTLV-1) Gag polyprotein. We found that this motif was required for efficient HTLV-1 infection, and that the specificity of SNX9 SH3 for the RxAPxxP core binding motif was importantly involved in this process.
Organizational Affiliation:
Department of Biological and Environmental Science, University of Jyvaskyla, Jyvaskyla FI-40014, Finland.