Broad and potent neutralizing human antibodies to tick-borne flaviviruses protect mice from disease.
Agudelo, M., Palus, M., Keeffe, J.R., Bianchini, F., Svoboda, P., Salat, J., Peace, A., Gazumyan, A., Cipolla, M., Kapoor, T., Guidetti, F., Yao, K.H., Elsterova, J., Teislerova, D., Chrdle, A., Honig, V., Oliveira, T., West, A.P., Lee, Y.E., Rice, C.M., MacDonald, M.R., Bjorkman, P.J., Ruzek, D., Robbiani, D.F., Nussenzweig, M.C.(2021) J Exp Med 218
- PubMed: 33831141
- DOI: https://doi.org/10.1084/jem.20210236
- Primary Citation of Related Structures:
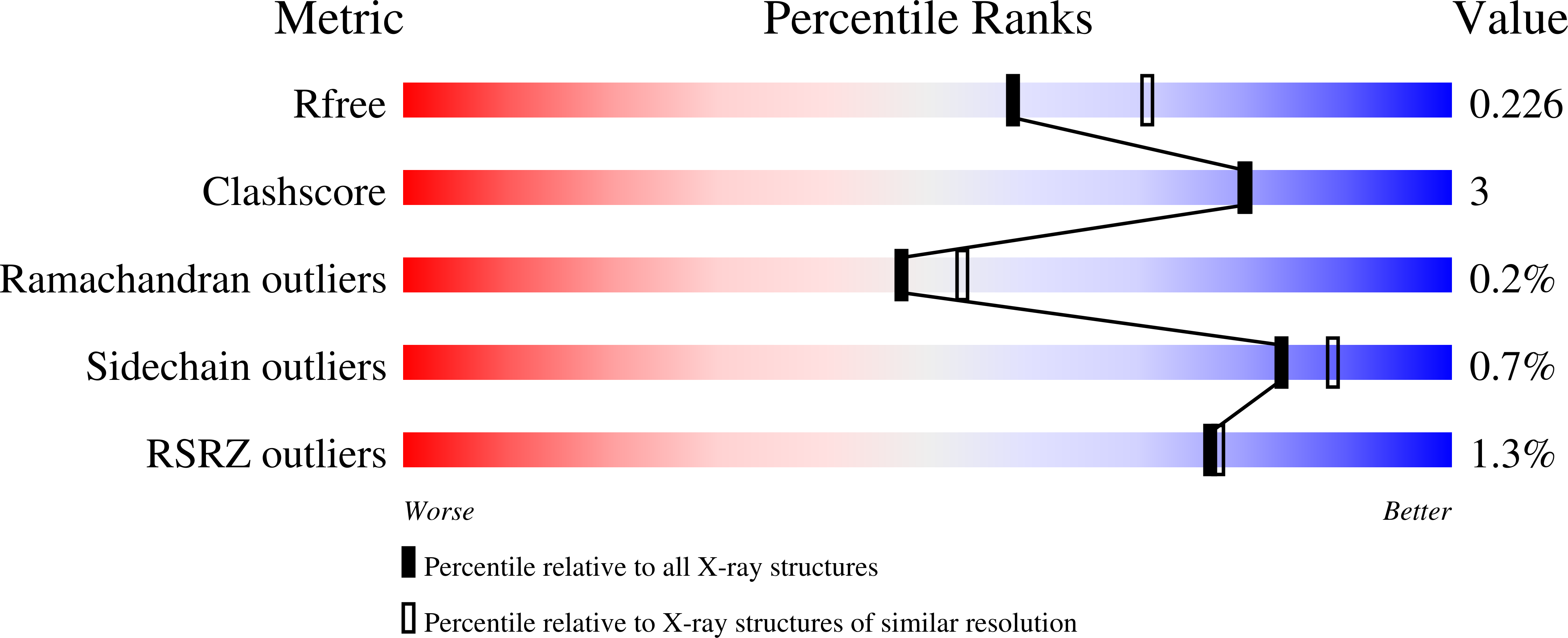
7LSE, 7LSF, 7LSG - PubMed Abstract:
Tick-borne encephalitis virus (TBEV) is an emerging human pathogen that causes potentially fatal disease with no specific treatment. Mouse monoclonal antibodies are protective against TBEV, but little is known about the human antibody response to infection. Here, we report on the human neutralizing antibody response to TBEV in a cohort of infected and vaccinated individuals. Expanded clones of memory B cells expressed closely related anti-envelope domain III (EDIII) antibodies in both groups of volunteers. However, the most potent neutralizing antibodies, with IC50s below 1 ng/ml, were found only in individuals who recovered from natural infection. These antibodies also neutralized other tick-borne flaviviruses, including Langat, louping ill, Omsk hemorrhagic fever, Kyasanur forest disease, and Powassan viruses. Structural analysis revealed a conserved epitope near the lateral ridge of EDIII adjoining the EDI-EDIII hinge region. Prophylactic or early therapeutic antibody administration was effective at low doses in mice that were lethally infected with TBEV.
Organizational Affiliation:
Laboratory of Molecular Immunology, The Rockefeller University, New York, NY.