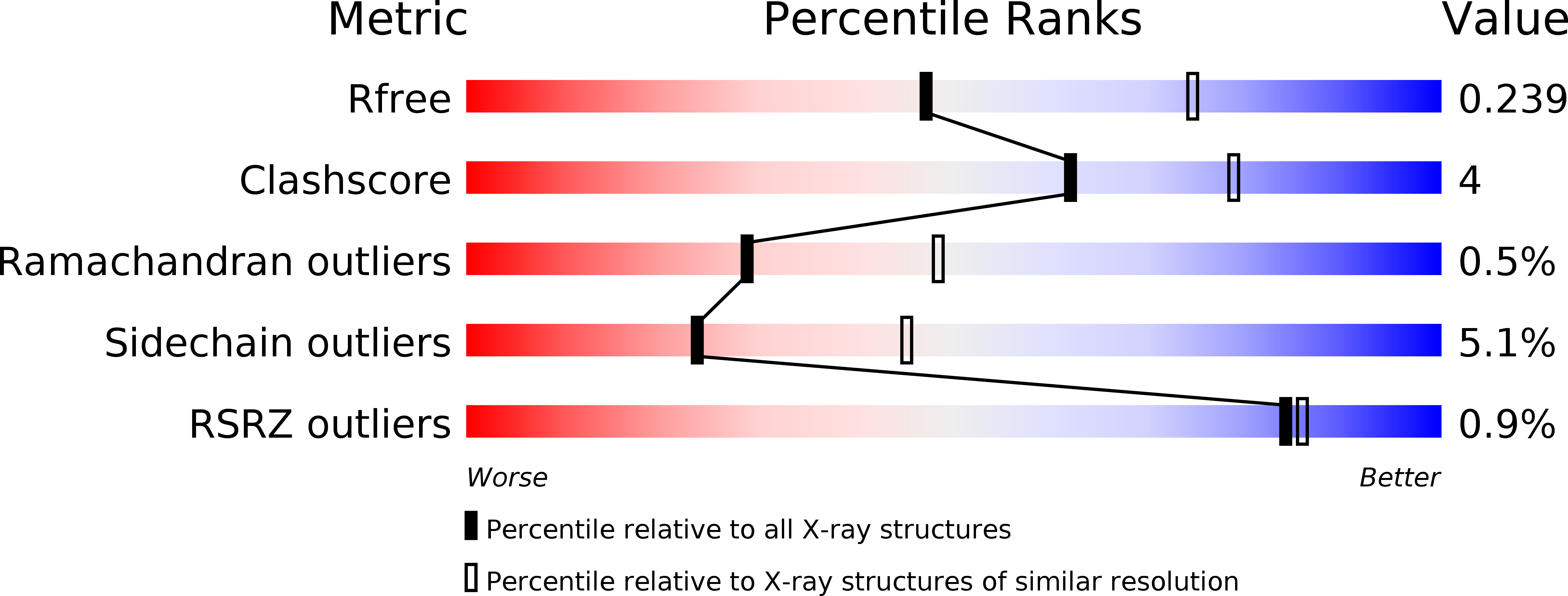
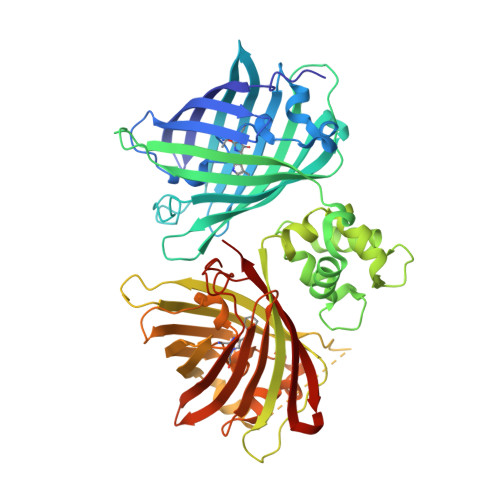
Dynamic tuning of FRET in a green fluorescent protein biosensor.
Trigo-Mourino, P., Thestrup, T., Griesbeck, O., Griesinger, C., Becker, S.(2019) Sci Adv 5: eaaw4988-eaaw4988
- PubMed: 31457088
- DOI: https://doi.org/10.1126/sciadv.aaw4988
- Primary Citation of Related Structures:
6GEL, 6GEZ - PubMed Abstract:
Förster resonance energy transfer (FRET) between mutants of green fluorescent protein is widely used to monitor protein-protein interactions and as a readout mode in fluorescent biosensors. Despite the fundamental importance of distance and molecular angles of fluorophores to each other, structural details on fluorescent protein FRET have been missing. Here, we report the high-resolution x-ray structure of the fluorescent proteins mCerulean3 and cpVenus within the biosensor Twitch-2B, as they undergo FRET and characterize the dynamics of this biosensor with
0 2 -dependent paramagnetic nuclear magnetic resonance at 900 MHz and 1.1 GHz. These structural data provide the unprecedented opportunity to calculate FRET from the x-ray structure and to compare it to experimental data in solution. We find that interdomain dynamics limits the FRET effect and show that a rigidification of the sensor further enhances FRET.
Organizational Affiliation:
Department for NMR-Based Structural Biology, Max Planck Institute for Biophysical Chemistry, Göttingen, Germany.