A Structure-Guided Switch in the Regioselectivity of a Tryptophan Halogenase.
Shepherd, S.A., Menon, B.R., Fisk, H., Struck, A.W., Levy, C., Leys, D., Micklefield, J.(2016) Chembiochem 17: 821-824
- PubMed: 26840773
- DOI: https://doi.org/10.1002/cbic.201600051
- Primary Citation of Related Structures:
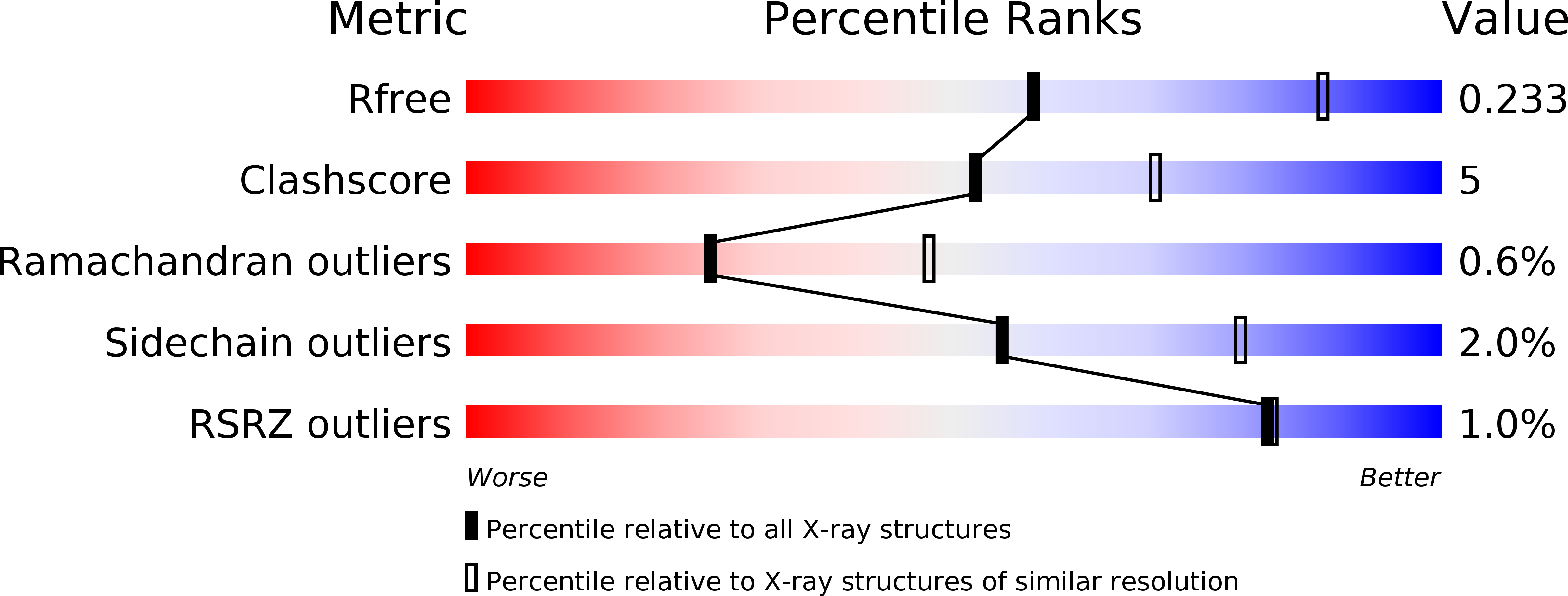
5HY5 - PubMed Abstract:
Flavin-dependent halogenases are potentially useful biocatalysts for the regioselective halogenation of aromatic compounds. Haloaromatic compounds can be utilised in the synthesis and biosynthesis of pharmaceuticals and other valuable products. Here we report the first X-ray crystal structure of a tryptophan 6-halogenase (SttH), which enabled key residues that contribute to the regioselectivity in tryptophan halogenases to be identified. Structure-guided mutagenesis resulted in a triple mutant (L460F/P461E/P462T) that exhibited a complete switch in regioselectivity; with the substrate 3-indolepropionate 75 % 5-chlorination was observed with the mutant in comparison to 90 % 6-chlorination for the wild-type SttH. This is the first clear example of how regiocomplementary halogenases can be created from a single parent enzyme. The biocatalytic repertoire of SttH was also expanded to include a range of indolic and non-indolic substrates.
Organizational Affiliation:
School of Chemistry and Manchester Institute of Biotechnology, The University of Manchester, 131 Princess Street, Manchester, M1 7DN, UK.