A Critical Base Pair in K-Turns Determines the Conformational Class Adopted, and Correlates with Biological Function.
Huang, L., Wang, J., Lilley, D.M.J.(2016) Nucleic Acids Res 44: 5390
- PubMed: 27016741
- DOI: https://doi.org/10.1093/nar/gkw201
- Primary Citation of Related Structures:
5FJ0, 5FJ1, 5FJ4, 5FJC, 5FK1, 5FK2, 5FK3, 5FK4, 5FK5, 5FK6, 5FKD, 5FKE, 5FKF, 5FKG, 5FKH - PubMed Abstract:
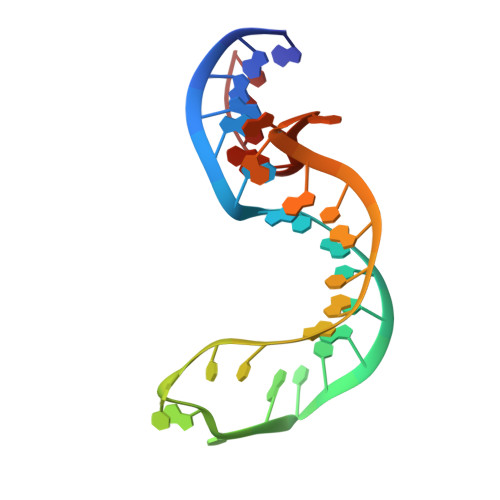
k-turns are commonly-occurring motifs that introduce sharp kinks into duplex RNA, thereby facilitating tertiary contacts. Both the folding and conformation of k-turns are determined by their local sequence. k-turns fall into two conformational classes, called N3 and N1, that differ in the pattern of hydrogen bonding in the core. We show here that this is determined by the basepair adjacent to the critical G•A pairs. We determined crystal structures of a series of Kt-7 variants in which this 3b,3n position has been systematically varied, showing that this leads to a switch in the conformation. We have previously shown that the 3b,3n position also determines the folding characteristics of the k-turn, i.e. whether or not the k-turn can fold in the presence of metal ions alone. We have analyzed the distribution of 3b,3n sequences from four classes of k-turns from ribosomes, riboswitches and U4 snRNA, finding a strong conservation of properties for a given k-turn type. We thus demonstrate a strong association between biological function, 3b,3n sequence and k-turn folding and conformation. This has strong predictive power, and can be applied to the modeling of large RNA architectures.
Organizational Affiliation:
Cancer Research UK Nucleic Acid Structure Research Group, MSI/WTB Complex, The University of Dundee, Dow Street, Dundee DD1 5EH, UK.