A novel transcriptional regulator of L-arabinose utilization in human gut bacteria.
Chang, C., Tesar, C., Li, X., Kim, Y., Rodionov, D.A., Joachimiak, A.(2015) Nucleic Acids Res 43: 10546-10559
- PubMed: 26438537
- DOI: https://doi.org/10.1093/nar/gkv1005
- Primary Citation of Related Structures:
5BS6, 5DD4, 5DDG, 5DEQ - PubMed Abstract:
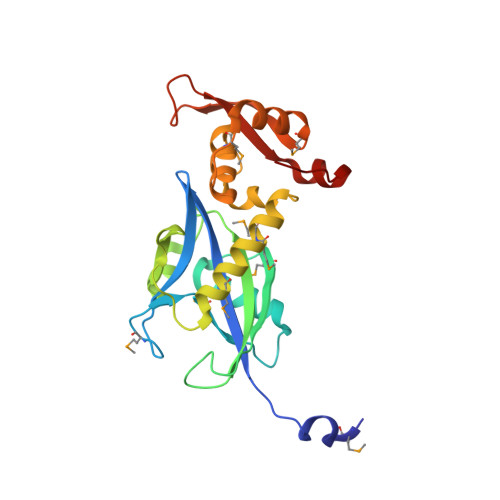
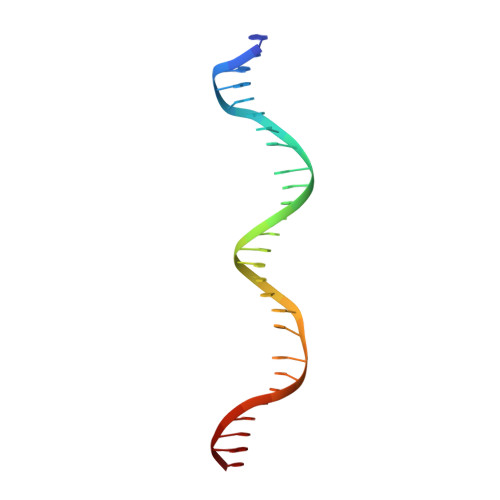
Carbohydrate metabolism plays a crucial role in the ecophysiology of human gut microbiota. Mechanisms of transcriptional regulation of sugar catabolism in commensal and prevalent human gut bacteria such as Bacteroides thetaiotaomicron remain mostly unknown. By a combination of bioinformatics and experimental approaches, we have identified an NrtR family transcription factor (BT0354 in B. thetaiotaomicron, BtAraR) as a novel regulator controlling the arabinose utilization genes. L-arabinose was confirmed to be a negative effector of BtAraR. We have solved the crystal structures of the apo and L-arabinose-bound BtAraR proteins, as well as the complex of apo-protein with a specific DNA operator. BtAraR forms a homodimer with each subunit comprised of the ligand-binding Nudix hydrolase-like domain and the DNA-binding winged-helix-turn-helix (wHTH) domain. We have identified the residues involved in binding of L-arabinose and recognition of DNA. The majority of these residues are well conserved in the AraR orthologs in Bacteroidetes. In the structure of the BtAraR-DNA complex, we found the unique interaction of arginine intercalating its guanidinum moiety into the base pair stacking of B-DNA. L-arabinose binding induces movement of wHTH domains, resulting in a conformation unsuitable for DNA binding. Our analysis facilitates reconstruction of the metabolic and regulatory networks involved in carbohydrate utilization in human gut Bacteroides.
Organizational Affiliation:
Midwest Center for Structural Genomics, Argonne National Laboratory, Argonne, IL 60439, USA Structural Biology Center, Biosciences Division, Argonne National Laboratory, Argonne, IL 60439, USA.