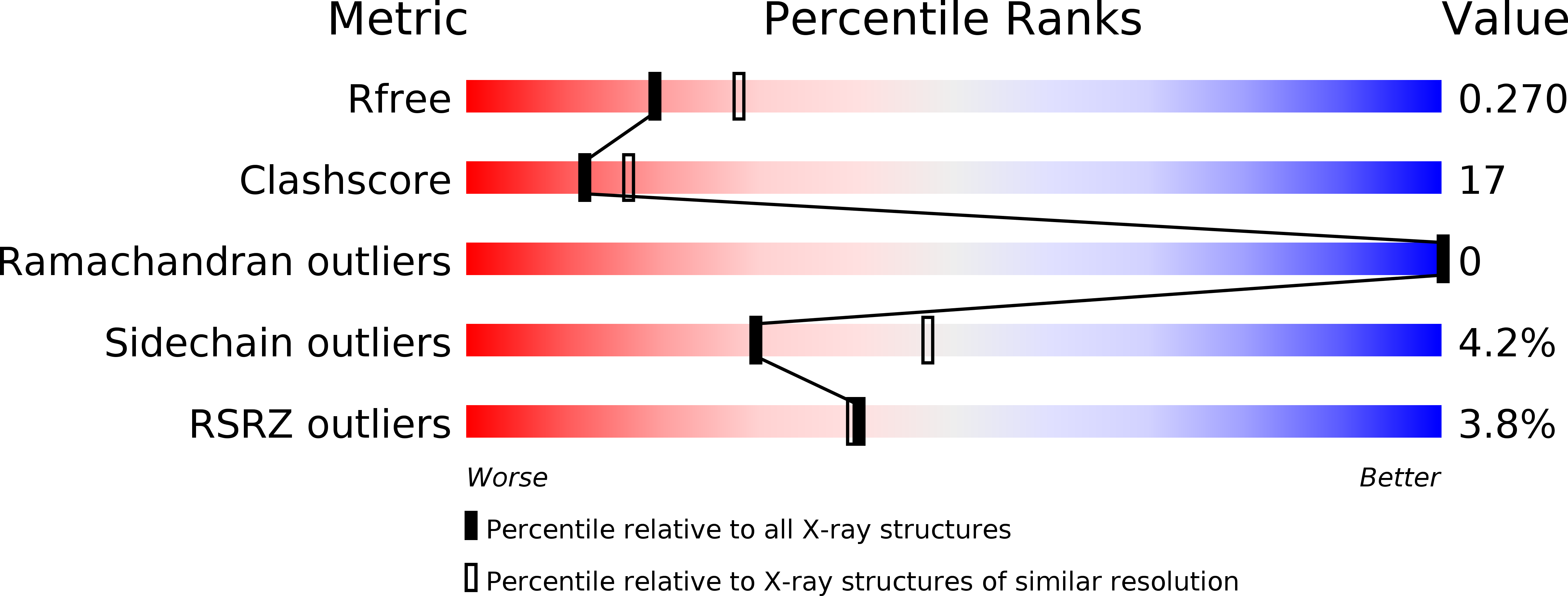
Hyperbolic Pressure-Temperature Phase Diagram of the Zinc-Finger Protein apoKti11 Detected by NMR Spectroscopy.
Klamt, A., Nagarathinam, K., Tanabe, M., Kumar, A., Balbach, J.(2019) J Phys Chem B 123: 792-801
- PubMed: 30608169
- DOI: https://doi.org/10.1021/acs.jpcb.8b11019
- Primary Citation of Related Structures:
5AX2 - PubMed Abstract:
For a comprehensive understanding of the thermodynamic state functions describing the stability of a protein, the influence of the intensive properties of temperature and pressure has to be known. With the zinc-finger-containing Kti11, we found a suitable protein for this purpose because folding and unfolding transitions occur at an experimentally accessible temperature (280-330 °K) and pressure (0.1-240 MPa) range. We solved the crystal structure of the apo form of Kti11 to reveal two disulfide bonds at the metal-binding site, which seals off a cavity in the β-barrel part of the protein. From a generally applicable proton NMR approach, we could determine the populations of folded and unfolded chains under all conditions, leading to a hyperbolic pressure-temperature phase diagram rarely observed for proteins. A global fit of a two-state model to all derived populations disclosed reliable values for the change in Gibbs free energy, volume, entropy, heat capacity, compressibility, and thermal expansion upon unfolding. The unfolded state of apoKti11 has a lower compressibility compared to the native state and a smaller volume at ambient pressure. Therefore, a pressure increase up to 200 MPa reduces the population of the native state, and above this value, the native population increases again. Pressure-induced chemical-shift changes in two-dimensional 1 H- 15 N NMR spectra could be employed for a molecular interpretation of the thermodynamic properties of apoKti11.
Organizational Affiliation:
Institute of Physics, Biophysics , Martin-Luther University Halle-Wittenberg , Betty-Heimann Street 7 , 06120 Halle , Germany.