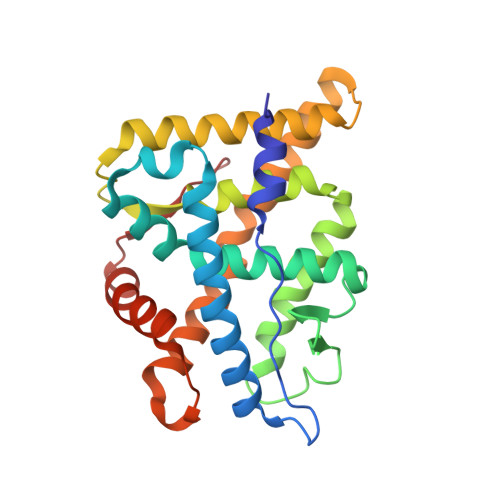
Structural Analysis of the Glucocorticoid Receptor Ligand-Binding Domain in Complex with Triamcinolone Acetonide and a Fragment of the Atypical Coregulator, Small Heterodimer Partner.
Weikum, E.R., Okafor, C.D., D'Agostino, E.H., Colucci, J.K., Ortlund, E.A.(2017) Mol Pharmacol 92: 12-21
- PubMed: 28396564
- DOI: https://doi.org/10.1124/mol.117.108506
- Primary Citation of Related Structures:
5UFS - PubMed Abstract:
The synthetic glucocorticoids (GCs) dexamethasone, mometasone furoate, and triamcinolone acetonide are pharmaceutical mainstays to treat chronic inflammatory diseases. These drugs bind to the glucocorticoid receptor (GR), a ligand-activated transcription factor and member of the nuclear receptor superfamily. The GR is widely recognized as a therapeutic target for its ability to counter proinflammatory signaling. Despite the popularity of GCs in the clinic, long-term use leads to numerous side effects, driving the need for new and improved drugs with less off-target pharmacology. X-ray crystal structures have played an important role in the drug-design process, permitting the characterization of robust structure-function relationships. However, steroid receptor ligand-binding domains (LBDs) are inherently unstable, and their crystallization requires extensive mutagenesis to enhance expression and crystallization. Here, we use an ancestral variant of GR as a tool to generate a high-resolution crystal structure of GR in complex with the potent glucocorticoid triamcinolone acetonide (TA) and a fragment of the small heterodimer partner (SHP). Using structural analysis, molecular dynamics, and biochemistry, we show that TA increases intramolecular contacts within the LBD to drive affinity and enhance stability of the receptor-ligand complex. These data support the emerging theme that ligand-induced receptor conformational dynamics at the mouth of the pocket play a major role in steroid receptor activation. This work also represents the first GR structure in complex with SHP, which has been suggested to play a role in modulating hepatic GR function.
Organizational Affiliation:
Department of Biochemistry, Emory University School of Medicine, Atlanta, Georgia.