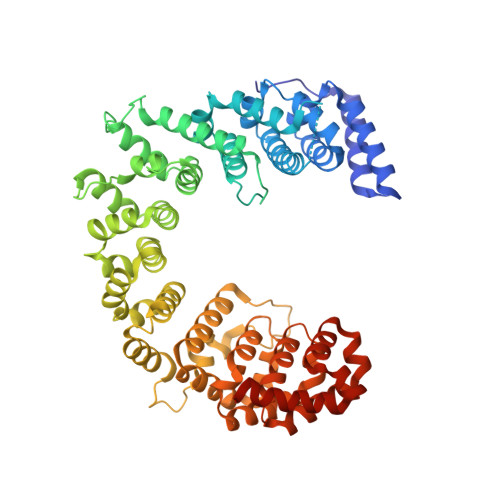
Nop9 is a PUF-like protein that prevents premature cleavage to correctly process pre-18S rRNA.
Zhang, J., McCann, K.L., Qiu, C., Gonzalez, L.E., Baserga, S.J., Hall, T.M.(2016) Nat Commun 7: 13085-13085
- PubMed: 27725644
- DOI: https://doi.org/10.1038/ncomms13085
- Primary Citation of Related Structures:
5SVD - PubMed Abstract:
Numerous factors direct eukaryotic ribosome biogenesis, and defects in a single ribosome assembly factor may be lethal or produce tissue-specific human ribosomopathies. Pre-ribosomal RNAs (pre-rRNAs) must be processed stepwise and at the correct subcellular locations to produce the mature rRNAs. Nop9 is a conserved small ribosomal subunit biogenesis factor, essential in yeast. Here we report a 2.1-Å crystal structure of Nop9 and a small-angle X-ray-scattering model of a Nop9:RNA complex that reveals a 'C'-shaped fold formed from 11 Pumilio repeats. We show that Nop9 recognizes sequence and structural features of the 20S pre-rRNA near the cleavage site of the nuclease, Nob1. We further demonstrate that Nop9 inhibits Nob1 cleavage, the final processing step to produce mature small ribosomal subunit 18S rRNA. Together, our results suggest that Nop9 is critical for timely cleavage of the 20S pre-rRNA. Moreover, the Nop9 structure exemplifies a new class of Pumilio repeat proteins.
Organizational Affiliation:
Epigenetics and Stem Cell Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, PO Box 12233, MD F3-05, Research Triangle Park, North Carolina 27709, USA.