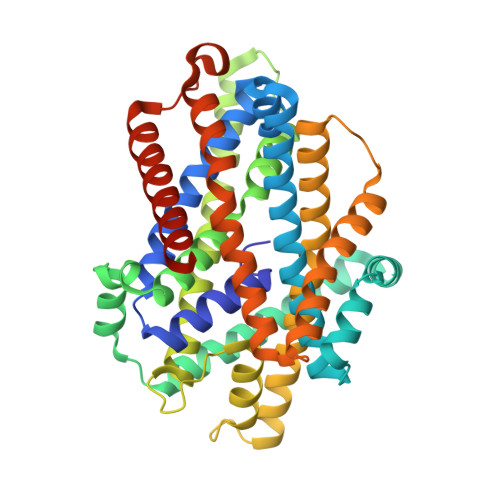
Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport.
Ehrnstorfer, I.A., Geertsma, E.R., Pardon, E., Steyaert, J., Dutzler, R.(2014) Nat Struct Mol Biol 21: 990-996
- PubMed: 25326704
- DOI: https://doi.org/10.1038/nsmb.2904
- Primary Citation of Related Structures:
5M94, 5M95 - PubMed Abstract:
Members of the SLC11 (NRAMP) family transport iron and other transition-metal ions across cellular membranes. These membrane proteins are present in all kingdoms of life with a high degree of sequence conservation. To gain insight into the determinants of ion selectivity, we have determined the crystal structure of Staphylococcus capitis DMT (ScaDMT), a close prokaryotic homolog of the family. ScaDMT shows a familiar architecture that was previously identified in the amino acid permease LeuT. The protein adopts an inward-facing conformation with a substrate-binding site located in the center of the transporter. This site is composed of conserved residues, which coordinate Mn2+, Fe2+ and Cd2+ but not Ca2+. Mutations of interacting residues affect ion binding and transport in both ScaDMT and human DMT1. Our study thus reveals a conserved mechanism for transition-metal ion selectivity within the SLC11 family.
Organizational Affiliation:
Department of Biochemistry, University of Zurich, Zurich, Switzerland.